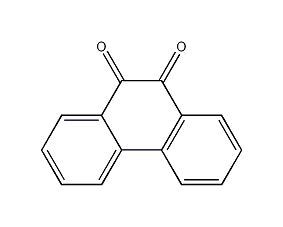
Structural formula
| Business number | 01UE |
|---|---|
| Molecular formula | C14H8O2 |
| Molecular weight | 208.21 |
| label |
9,10-phenanthrenequinone, 9,10-phenanthrenedione, phenanthrenequinone, 9,10-Phenanthrenedione, Aromatic aldehydes, ketones and their derivatives |
Numbering system
CAS number:84-11-7
MDL number:MFCD00001163
EINECS number:201-515-5
RTECS number:SF7875000
BRN number:608838
PubChem ID:None
Physical property data
1. Properties: Orange-yellow needle-like crystals.
2. Density (g/mL, 25/4℃): 1.405
3. Relative density (20℃, 4℃): 1.40522
4. Melting point (ºC): 209℃
5. Boiling point (ºC, normal pressure): above 360℃
6. Boiling point (ºC, 5.2 kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º ): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, slightly Soluble in ethanol, benzene, ethyl acetate, soluble in ether and hot acetic acid
Toxicological data
Acute toxicity:
Mouse abdominal cavity LD50: 165mg/kg;
2. Chronic toxicity/carcinogenicity: Mouse skin TDL0: 800 mg/kg/29W-C
3. Teratogenicity
Salmonella: 30 umol/L;
Bacillus subtilis: 400ug/plate
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 58.66
2. Molar volume (cm3/mol): 159.0
3. Isotonic specific volume (90.2K ): 434.8
4. Surface tension (dyne/cm): 55.8
5. Polarizability (10-24cm3): 23.25
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Topological molecular polar surface area (TPSA): 34.1
6. Number of heavy atoms: 16
7. Surface charge: 0
8. Complexity: 289
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters: 0
11. No Determine the number of atomic stereocenters: 0
12, Determine the number of chemical bond stereocenters: 0
13, Uncertain number of chemical bond stereocenters: 0
14. Number of covalent bond units: 1
Properties and stability
This product can be absorbed through the skin and cause poisoning. Animal test results show that the lowest oral poisoning dose for mice is 20 mg/kg (for one consecutive day). Operators should wear protective equipment.
Storage method
This product should be sealed and stored in a cool, dry place.
Packed in plastic bags lined with woven bags, stored in a cool, dry and ventilated place, protected from sun and moisture, and away from fire and heat sources. Store and transport according to general chemical regulations.
Synthesis method
Made from phenanthrene oxidation, there are chemical methods and electrolysis methods. 1. Chemical method uses sodium (or potassium) dichromate and phenanthrene in sulfuric acid aqueous solution for oxidation. 2. The electrolyte of the electrolytic oxidation method contains 120g/L of chromium trioxide, 450g/L of sulfuric acid, and 35g/L of phenanthrene. The cathode current density is 3.75A/cm2, the electrolyte temperature is 60-65°C, and after 8 hours of electrolysis, the product is filtered.
![]()
Purpose
Phenanthrenequinone itself is a pesticide and can be used as a fungicide, seed dressing agent, electrophotography, photoconductive material, photosensitive solder resist and paper preservative.

 微信扫一扫打赏
微信扫一扫打赏

