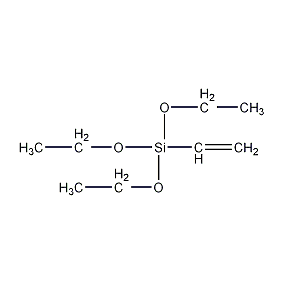
Structural formula
| Business number | 01MP |
|---|---|
| Molecular formula | C8H18O3Si |
| Molecular weight | 190.31 |
| label |
Triethoxyethylenesilane, Triethoxy vinyl silane, Coupling agent |
Numbering system
CAS number:78-08-0
MDL number:MFCD00009063
EINECS number:201-081-7
RTECS number:VV6700000
BRN number:1767229
PubChem number:24890089
Physical property data
1. Properties: Colorless transparent liquid
2. Density (g/mL, 20℃): 0.93
3. Melting point (ºC): <0
4. Boiling point (ºC, normal pressure): 160.5
5. Refractive index (25℃): 1.3961
6. Flash point (℃): 54
p>
7. Solubility: Insoluble in water, miscible in alcohol, ether and benzene
Toxicological data
1. Skin/eye irritation
Open irritation test: rabbit, skin contact: 500mg, severity of reaction: mild.
Standard Draize test: Rabbit, eye contact: 500mg/24H; severity of reaction: mild.
2. Acute toxicity: rat oral LD50: 8mL/kg; rat inhalation LC50: 4000ppm/4H; rat abdominal LD50: 5700μL/kg; mouse inhalation LC50: 23mg/ m3/4H; Rabbit skin contact LD50: 10mL/kg
Ecological data
Slightly hazardous to water, avoid contact of undiluted or large quantities of product with groundwater, waterways or sewage systems.
Molecular structure data
1. Molar refractive index: 52.78
2. Molar volume (cm3/mol): 210.5
3. Isotonic specific volume (90.2K): 457.5
4. Surface tension (dyne/cm): 22.3
5. Polarizability (10-24cm3): 20.92
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 7
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 27.7
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 111
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters : 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Reacts with strong oxidants, strong bases, water, and moist air.
NoneColorful liquid, soluble in ethanol, isopropyl alcohol, benzene, toluene and gasoline, insoluble in water. Mixed with acid aqueous solution to hydrolyze and dissolve in water.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, alkalis, etc. and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
It can be prepared by the reaction of vinyl trichlorosilane and absolute ethanol, or by the reaction of tetraethoxysilane and vinyl magnesium bromide.
1. Synthesis of Vinyl Trichlorosilane: Heat trichlorosilane and then pass it into acetylene, preferably in the liquid phase in the presence of a catalyst such as peroxide, tertiary amine or platinum salt. Or it can be obtained by addition reaction in gas phase.

2. Vinyl triethyl Synthesis of silane
![]()
![]()
3.Third B Oxyhydrogen silicon and acetylene are used as raw materials, and the product is obtained by one-step addition under the catalytic action of dichlorobistriphenylphosphonium and platinum salt. The reaction temperature is 90~130℃, and the synthesis reaction formula is as follows:![]()
There are two processes: liquid phase method and gas phase method. The so-called liquid phase method is to add triethoxysilane and catalyst into the reaction system together, and then pass acetylene into the reaction material for reaction. In the gas phase method, the catalyst is loaded into a tubular reactor, and then triethoxysilane is vaporized, mixed with acetylene, and introduced into the reactor for reaction.
can also be synthesized first Vinyltrichlorosilane, and then use vinyltrichlorosilane and ethanol to react by heating to obtain vinyltriethoxysilane; or vinyltrichlorosilane and triethyl orthoformate in the presence of ethanolPrepared by co-heating esters together: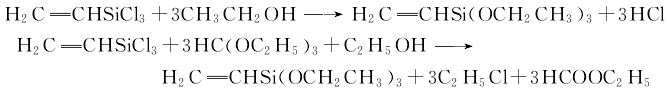
Purpose
1. It has the functions of both coupling agent and cross-linking agent. Applicable polymer types include polyethylene, polypropylene, unsaturated polyester, etc., which are commonly used in fiberglass, plastics, glass, cables, ceramics, rubber, etc. ; Used as an intermediate for silicone.
2.Coupling agent suitable for unsaturated polyester, acrylic resin, ethylene-propylene rubber and their fillers, which can improve the relationship between fillers and rubber adhesive properties. It is also used as a glass fiber treatment agent to improve the bonding and wetting properties of acrylic resin, unsaturated polyester, polyethylene, polypropylene and other resins with glass fiber, improve the mechanical strength of glass fiber reinforced plastics, and also improve its water resistance and resistance to water. Thermal, weather resistance and electrical properties. It is also used for insulation and moisture-proof treatment of radio parts.

 微信扫一扫打赏
微信扫一扫打赏

