Background and overview[1]
O-bromophenylpropionaldehyde can be used as a pharmaceutical synthesis intermediate. If o-bromophenylpropionaldehyde is inhaled, please move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel unwell; if the eye contact occurs, seek medical attention. Separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
O-bromophenylpropionaldehyde can be used as a pharmaceutical synthesis intermediate. For example, prepare the following compounds:
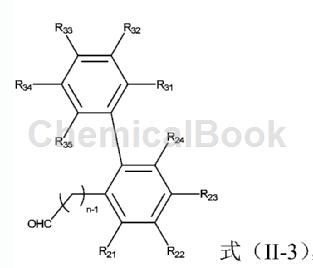
Wherein: R11, R12 and R13 are each independently selected from H, halogen, C1-4 alkyl and C1-4 alkyl substituted by 1-3 halogens; R21, R22, R23 and R24 are each independently selected is selected from H, halogen, C1-6 alkyl, C1-6 alkoxy, C1-6 alkyl substituted by 1-3 halogens and C1-6 alkyl substituted by 1-3 halogens Oxygen; R31, R32, R33, R34 and R35 are each independently selected from H, halogen, C1-6 alkyl, C1-6 alkoxy, C1-6 alkyl substituted by 1-3 halogens and a C1-6 alkoxy group substituted by 1-3 halogens; R4 is a C1-6 alkyl group, a C1-6 alkoxy group; n is an integer of 1-6.
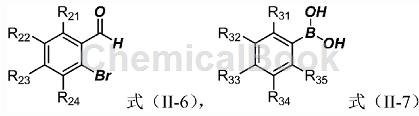
The specific steps are: In a 100mL eggplant-shaped bottle, add o-bromobenzaldehyde (4mmol) represented by formula (II-6), then add 12mL of acetonitrile, and add in sequence represented by formula (II-7) while stirring. Phenylboronic acid (6mmol), potassium fluoride (10.4mmol), bis(triphenylphosphine)palladium dichloride (28mg), add 4mL of water, and heat to reflux. When the solution turns from yellow to red-black, use TLC spot plate to monitor whether the reaction is complete. If the reaction is complete, filter it with diatomaceous earth after cooling, and directly mix the sample with silica gel and pass it through the column to obtain a colorless or yellowish solid. The yield is basically maintained at Around 85%.
Main reference materials
[1] (CN109422691) N-substituted amide compounds and their preparation methods and applications and fungicides

 微信扫一扫打赏
微信扫一扫打赏

