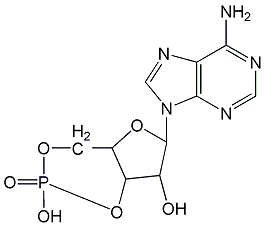
Structural formula
| Business number | 01BP |
|---|---|
| Molecular formula | C10H12N5O6P |
| Molecular weight | 329.21 |
| label |
cyclic adenosine monophosphate, cyclic adenosine monophosphate, 3′,5′-cyclic adenosine; adenine riboside-3′,5′-cyclic phosphate, 3′,5′-Cyclic AMP, Adenosine 3′,5′-cyclophosphate, cAMP, Acrasin, Adeosine-3′,5′-cyclic, protein kinase activator |
Numbering system
CAS number:60-92-4
MDL number:MFCD00005845
EINECS number:200-492-9
RTECS number:AU7357600
BRN number:52645
PubChem number:24278245
Physical property data
1. Properties: White pearlescent flake crystals.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 219-220
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º, c= 0.67 g/ml): -53.7
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
p>
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC ): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Hygroscopic. Stable indoors. Slightly soluble in water, almost insoluble in ethanol or ether.
Toxicological data
1. Mutagenicity: Mutation test system – not other specifiedTEST system: human cells – not otherwise specified: 100umol/L; Mutation test system – not other specifiedTEST system: rodent – mouse liver: 10nmol/L; Mutation test System – Not Other SpecifiedTEST System: Rodent – Mouse Cells – Not Otherwise Specified: 10nmol/L; DNA inhibitionTEST System: Rodent – Mouse Cells – Not Otherwise Specified: 10nmol/L; Mutation Test System – Not Other SpecifiedTEST Systems: Rodent – Mouse Fibroblasts: 500umol/L
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 67.04
2. Molar volume (cm3/mol): 133.0
3. Isotonic specific volume (90.2K): 468.6
4. Surface tension (dyne/cm): 153.8
5. Polarizability (10-24cm3): 26.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -2.6
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 10
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecular polar surface area (TPSA): 155
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 498
10. Isotopes Number of atoms: 0
11. Determine the number of atomic stereocenters: 4
12. Uncertain number of atomic stereocenters: 0
13. Determine chemical bonds Number of stereocenters: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
It has a regulatory effect on many enzyme-catalyzed reactions. It can regulate the activity of a series of enzymes that react with sugars and fats stored in cells. It also regulates protein biosynthesis. effect. It can be widely involved in the regulation of cell functions. It can relax smooth muscles, dilate blood vessels, improve liver function, promote nerve regeneration, inhibit the division of outer skin cells and transform the function of abnormal cells, promote the activity of respiratory chain oxidases, improve myocardial hypoxia, etc.
Storage method
Seal and store in a dry place below 0℃ and away from light.
Synthesis method
Using 5′-AMP as raw material
The preparation ratio of 5′-AMP double salt is N, N’-dicyclohexylmorphine (m): pyridine (V): 5′-AMP (m) : Water (V)=1:9.62:1.19:1.7. Add N, N’-dicyclohexylmorpholine and pyridine into the reactor, heat to 80°C to dissolve, add 5′-AMP and water, after 5′-AMP is dissolved, distill under reduced pressure at 80°C until dry. Add a small amount of anhydrous pyridine, evaporate to dryness under reduced pressure, repeat twice to obtain the product.
5′-AMP[N,N’-dicyclohexylmorpholin, pyridine]→[80℃]5′-AMP double salt
The preparation and feeding ratio of crude cAMP is 5′-AMP complex Salt (m): anhydrous pyridine (V): dicyclohexylcarbodiimide (V)=1:235:1.07. First, mix one-half amount of anhydrous pyridine and dicyclohexylcarbodiimide, reflux at 140-145°C, and mix 5′-AMP double salt with another half amount of anhydrous water under reflux. Add the pyridine mixed solution slowly in batches, and finish the addition in about 3 hours. After the addition, continue to reflux for 6 hours, cool, and evaporate the pyridine under reduced pressure to dryness at 70°C. Dissolve the residue with a mixture of diethyl ether and water (1:1.5). Filter to remove the insoluble matter dicyclohexyl urea, separate the water layer in the filtrate, wash it three times with diethyl ether, and pump away the remaining diethyl ether with a water pump.
5′-AMP double salt [anhydrous pyridine, dicyclohexylcarbodiimide]→[9h]cAMP crude product
Purification of cAMP crude product Adjust the pH of the aqueous layer to 2 with hydrochloric acid, and filter to remove insoluble matter The filtrate was adsorbed with 100-mesh strong alkaline styrene-based cation exchange resin 001×7 (732), and the amount of resin was 80-100 times that of 5′-AMP. Then use 1.01mol/L hydrochloric acid to elute, collect the second absorption peak (E260), neutralize it with ammonium bicarbonate to neutrality, concentrate under reduced pressure at 60°C until the cAMP content is 15%-20%, filter, add the filtrate, etc. volume of ethanol (95%), adjust the pH to 2 with 2mol/L hydrochloric acid, filter, and dry to obtain the finished cAMP product.
cAMP crude product [001×7(732) resin, hydrochloric acid, ethanol] → [pH2] cAMP finished product.
Purpose
1. Activate inactive glycogen phosphatase in the liver and cause the adrenal cortex to produce hormones. 2. Protein kinase activator. It has the effects of improving myocardial hypoxia, dilating coronary arteries, enhancing myocardial contractility, and increasing cardiac output. It is used as an auxiliary treatment for angina pectoris and acute myocardial infarction, but its effect is short-lived. Occasionally, fever and rash may occur.

 微信扫一扫打赏
微信扫一扫打赏

