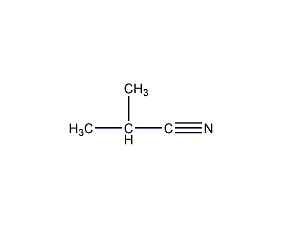
Structural formula
| Business number | 01NN |
|---|---|
| Molecular formula | C4H7N |
| Molecular weight | 69 |
| label |
isopropyl cyanide, Isopropylnitrile, 2-cyanopropane, Isopropyl cyanide, Isopropylnitrile, 2-Cyanopropane, synthetic raw materials |
Numbering system
CAS number:78-82-0
MDL number:MFCD00001873
EINECS number:201-147-5
RTECS number:TZ4900000
BRN number:1340512
PubChem number:24878313
Physical property data
1. Properties: colorless liquid with foul odor. [1]
2. Melting point (℃): -72[2]
3. Boiling point (℃): 103.8~104[3]
4. Relative density (water=1): 0.76 (30℃)[4]
5. Relative vapor density (air=1): 2.38[5]
6. Saturated vapor pressure (kPa): 13.3 (54.4℃)[6 ]
7. Heat of combustion (kJ/mol): -2559.8[7]
8. Critical pressure (MPa): 3.76 [8]
9. Octanol/water partition coefficient: 0.46[9]
10. Flash point (℃ ): 8 (CC) [10]
11. Ignition temperature (℃): 482[11]
12. Solubility: Slightly soluble in water, easily soluble in most organic solvents such as ethanol and ether. [12]
13. Viscosity (mPa·s): 0.456
14. Refractive index (20ºC): 1.3720
15. Heat of evaporation (KJ/mol): 35.38
16. Heat of fusion (KJ/mol): -12.23
17. Heat of formation (KJ/mol): -12.22
18. Heat of combustion (KJ/mol): -2564.0
Toxicological data
1. Acute toxicity: rat caliber LD50: 50mg/kg; mouse inhalation LC50: 100PPM/4H; mouse caliber LD50: 25mg/kg; mouse abdominal cavity LD50: 25mg/kg; rabbit caliber LD50: 14mg/ kg; rabbit skin LD50: 200mg/kg; rabbit subcutaneous LDL0: 9mg/kg; pig skin LDL0: 5mg/kg; frog subcutaneous LDL0: 4800mg/kg;
2. Neurotoxicity: Rabbit skin test: 380mg/24H;
3. It is moderately toxic. If the vapor is inhaled at 20℃, mice will die within two minutes and rats will die within 10 minutes. The symptoms of acute poisoning in animals are similar to those of other nitriles, including weakness, vasodilation, tremors and convulsions, significant respiratory depression, and increased urinary thiocyanate.
4. Acute toxicity [13] LD50: 50mg/kg (rat oral); 200mg/kg (rabbit dermal)
5. Irritation[14] Rabbit transdermal: 380mg, mild irritation (open stimulation test)
6. Subacute and chronic toxicity[15] Rat exposure to 1/5 or 1/10 LD50, once a day, 2 weeks , autopsy revealed liver parenchymal degeneration. Poisoning manifests as weakness, vasodilation, tremors, convulsions, and significant respiratory depression. Increased urinary thiocyanate excretion.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[16] MITI-I test, initial concentration 100ppm , the sludge concentration is 30ppm, and the degradation is 53.9~66.3% after 2 weeks.
3. Non-biodegradability[17] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 23d (theoretical).
4. Other harmful effects [18] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 20.45
2. Molar volume (cm3/mol): 88.3
3. Isotonic specific volume (90.2K ): 196.9
4. Surface tension (dyne/cm): 24.7
5. Polarizability (10-24cm3): 8.10
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 23.8
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 55.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[19] Stable
2. Incompatible substances[20] Strong oxidizing agent, strong reducing agent, strong acid, strong alkali
3. Polymerization hazard[21] No polymerization
Storage method
Storage Precautions[22] Storage in a cool, well-ventilated special warehouse, and implement the “two people to receive and receive, two people to keep” system. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, alkalis, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
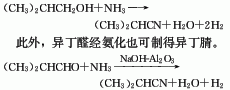
Isobutyronitrile can be synthesized from isobutanol and ammonia. Isobutanol and ammonia are synthesized in a fixed bed tubular reactor in a 1:2.5 ratio in the presence of a zinc oxide catalyst at a temperature of 410-450°C and normal pressure. After nitrification, they are cooled by water and ice-brine water. , isobutanol and water two-step absorption, ammonia analysis and other processes are produced. The conversion rate of isobutanol is close to 100%, the selectivity is over 90%, and the reaction yield is over 90%. The catalyst composition is zinc oxide and clay, and the ratio is 75:25. The catalyst has a service life of 1000 hours and can continue to be used after air activation. This method has a short process flow, simple equipment, high yield, and easy three-waste treatment. It is currently the main method for the industrial production of isobutyronitrile. There are also various preparation methods: dehydrogenation of isobutylamine under the catalysis of zinc sulfate; reaction of acetone and hydrocyanic acid to obtain cyanohydrin, which is then dehydrated and hydrogenated; reaction of propylene and hydrogen cyanide; reaction of isobutyraldehyde and ammonia, etc. , can prepare isobutyronitrile.
Refining method: use a small amount of concentrated hydrochloric acid Shake together to remove isonitrile. Wash with water and sodium bicarbonate aqueous solution. Or after drying with silica gel or type 4A molecular sieve, add calcium hydride and stir until hydrogen gas is no longer released. Pour off the liquid and then distill it in the presence of phosphorus pentoxide (the amount of phosphorus pentoxide should not exceed 5g/L). The distillate is refluxed with calcium hydride and slowly distilled in the presence of calcium hydride to obtain pure product. Pay attention to prevent moisture from entering during operation.
Purpose
1. Organic synthesis intermediates, mainly used in the production of isobutamidine, an intermediate of the organophosphorus pesticide diazinon. Also used as an additive in the production of acrylic resins.
2. Used in the production of pesticides and organic synthesis. [23]

 微信扫一扫打赏
微信扫一扫打赏

