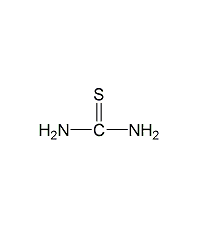
Structural formula
| Business number | 01CG |
|---|---|
| Molecular formula | CH4N2S |
| Molecular weight | 76 |
| label |
thiourea, Thiourea, thiocarbonamide, thiocarbamide, Sulfourea, aliphatic sulfur compounds, Organic corrosion inhibitor materials |
Numbering system
CAS number:62-56-6
MDL number:MFCD00008067
EINECS number:200-543-5
RTECS number:YU2800000
BRN number:605327
PubChem number:24889147
Physical property data
1. Character: white bright bitter crystal [1]
2. Melting point (℃): 182[2]
3. Boiling point (decomposition, ℃): 263[3]
4. Relative density (water = 1): 1.41[4]
5. Critical pressure (MPa): 8.23[5]
6. Octanol/water partition coefficient: -1.08~-1.02[6]
7. Flash point (℃): >182[7]
8. Solubility: soluble in cold water , ethanol, slightly soluble in ether. [8]
Toxicological data
1. Acute toxicity[9] LD50: 125mg/kg (rat oral); 100mg/kg (mouse abdominal cavity)
2. Irritation[10] Rabbit Meridian: 14%, causes irritation.
3. Mutagenicity[11] Microbial mutagenicity: Salmonella typhimurium 150μg/dish; Saccharomyces cerevisiae Bacteria 52600μmol/L.
4. Teratogenicity[12] Rats were given the lowest toxic dose (TDLo) orally 12 days after pregnancy 240mg/kg, causing developmental malformations of the central nervous system and musculoskeletal system. The lowest toxic dose (TDLo) of 1400mg/kg was administered orally to rats on the 16th to 22nd day after pregnancy, causing developmental malformation of the endocrine system.
5. Carcinogenicity[13] IARC Carcinogenicity Comment: G3, insufficient evidence of carcinogenicity to humans and animals .
6. Others[14] The lowest oral toxic dose in rats (TDLo): 40mg/kg (pregnant women) 1 day after administration), it affects the central nervous system, muscle and skeletal systems of fetal rats.
Ecological data
1. Ecotoxicity[15] LC50: >100mg/L (96h) (fathead minnow); 1.8mg/L (48h) (water fleas)
2. Biodegradability[16] MITI-I test, initial concentration 100ppm, sludge concentration 30ppm, degradation 2.6% after 2 weeks.
3. Non-biodegradability[17] In the air, when the hydroxyl radical concentration is 5.00×105 pieces/cm3, the degradation half-life is 0.4d��After the emulsion is absorbed, a calcium hydrosulfide solution is generated, and its content is controlled to be no less than 16.5%. Synthesis of thiourea: Add 160g of 16.5% Ca(SH)2 solution into a three-necked flask, start stirring, raise the temperature to about 50°C, and slowly add nitrogen 22% solid Ca(CN)2 277g, heated to 85°C, kept at constant temperature for 3 hours, the reaction was completed. Preparation of finished thiourea: Filter the above solution, wash the filter cake repeatedly with distilled water, combine the washing liquid and the filtrate, and evaporate under vacuum at 80~85℃ , evaporate until the content is 13~14°Bé. Cool the concentrated liquid to about 10°C, crystallize for 10 hours, and filter. The filtrate is repeatedly evaporated, crystallized, and filtered, and the filter cake is dried at 75°C to obtain the finished product. 6.The main synthesis methods are: reaction of cyanamides and thiols; reaction of amines and carbon disulfide; reaction of isothiocyanates and amines; Ammonium thiocyanate heating, etc. Germany was the first to use ammonium thiocyanate. Reynolds discovered in 1869 that this reaction is reversible. Industrial scale manufacturing began in the United States in 1940. Although many patents have been applied for, the main patent is the reaction of calcium cyanamide and hydrogen sulfide. ![]() 7. It can also be produced by ammonium thiocyanate method and diazomethane method .
7. It can also be produced by ammonium thiocyanate method and diazomethane method . 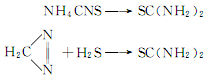
Purpose
1. This product is used as a raw material for the synthesis of sulfathiazole, methionine, fat pig tablets and other drugs. Used as raw material for dyes and dyeing auxiliaries, resins and compression molding powder. It can also be used as a vulcanization accelerator for rubber, a flotation agent for metal minerals, a catalyst for the preparation of phthalic anhydride and fumaric acid, and as a metal rust inhibitor. In terms of photographic materials, it can be used as a developer and toner. It can also be used in the electroplating industry. Thiourea is also used in diazo photosensitive paper, synthetic resin coatings, anion exchange resins, germination accelerators, fungicides and many other aspects. Thiourea is also used as a fertilizer. Pharmaceutically used as an intermediate in the production of drugs. Used as vulcanization accelerator in the rubber industry. Used as flotation agent in mining industry. It is also used as fabric and paper treatment agent, printing and dyeing auxiliary.
2. Analysis reagents. Used for the determination of bismuth, osmium, rhodium, selenium, lead, tellurium, nitrite, etc. Complex indicator for determination of osmium.
3. Used to prepare epoxy resin rapid curing agent. It can also be used as a toughening agent for melamine formaldehyde resin adhesives. In the pharmaceutical industry, it is used in the manufacture of sulfathiazole, methionine, piperic acid and antithyroid drugs. Used in organic synthesis to produce thiourea derivatives such as methyl thiourea, diethyl thiourea, diphenyl thiourea, and thiourea dioxide. The pesticide industry is used to manufacture insecticides. It is also used as reducing agent for azo photosensitive paper, metal rust inhibitor, processing aid for nylon and polyester fiber, rubber vulcanization accelerator, mineral flotation agent, organic synthesis catalyst, electroplating additive, etc.
4. Used as hydrofluoric acid pickling corrosion inhibitor and pickling copper remover.
5. In nitrilotriacetic acid zinc plating, thiourea can be used as a brightener, and it can also improve cathodic polarization, make the coating glossy, crystallize finely, and improve the throwing ability of the solution.
6. Used in organic synthesis, and also used as medicines, rubber accelerators, gold plating materials, etc. [25]

 微信扫一扫打赏
微信扫一扫打赏

