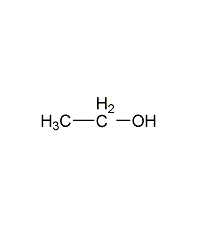
Structural formula
| Business number | 01D6 |
|---|---|
| Molecular formula | C2H6O |
| Molecular weight | 46.07 |
| label |
waterfree alcohol, Alcohol, fire wine, absolute ethanol, Ethyl alcohol, Anhydrous alcohol, Ethylhydrate, rubber additives, antifreeze, fuel, disinfectant, alcohol solvents, Aliphatic alcohols, ethers and their derivatives |
Numbering system
CAS number:64-17-5
MDL number:MFCD00003568
EINECS number:200-578-6
RTECS number:KQ6300000
BRN number:1718733
PubChem number:24872843
Physical property data
1. Properties: colorless liquid with wine aroma. [1]
2. Melting point (℃): -114.1[2]
3. Boiling point (℃): 78.3[3]
4. Relative density (water=1): 0.79 (20℃)[4]
5. Relative vapor density (air = 1): 1.59[5]
6. Saturated vapor pressure (kPa): 5.8 (20℃)[6]
7. Heat of combustion (kJ/mol): -1365.5[7]
8. Critical temperature (℃): 243.1[8]
9. Critical pressure (MPa): 6.38[9]
10. Octanol/water partition coefficient: 0.32[10]
11. Flash point (℃): 13 (CC); 17 (OC) [11]
12. Ignition temperature (℃): 363[12]
13. Explosion upper limit (%): 19.0[13]
14. Lower explosion limit (%): 3.3[14]
15. Solubility: miscible with water, miscible with ether, chloroform, and glycerin , methanol and most other organic solvents. [15]
16. Viscosity (mPa·s, 15ºC): 0.6405
17. Viscosity (mPa·s, 20ºC): 0.5945
18. Viscosity (mPa·s, 25ºC): 0.5525
19. Viscosity (mPa·s, 30ºC): 0.5142
20. Flash point (ºC, Open): 16.0
21. Flash point (ºC, closed): 14.0
22. Heat of evaporation (KJ/mol, b.p.): 38.95
23 . Heat of fusion (KJ/kg): 104.7
24. Heat of formation (KJ/mol, liquid): -277.8
25. Specific heat capacity (KJ/(kg·K), 20ºC, constant pressure): 2.42
26. Boiling point rising constant: 1.03~1.09
27. Conductivity (S/m): 1.35×10-19
28. Thermal conductivity (W/(m·K)): 18.00
29. Volume expansion coefficient (K-1, 20ºC ): 0.00108
30. Critical density (g·cm-3): 0.275
31. Critical volume (cm3·mol-1): 168
32. Critical compression factor: 0.241
33. Eccentricity factor: 0.637
34 .Lennard-Jones parameter (A): 4.5564
35. Lennard-Jones parameter (K): 424.51
36. Solubility parameter (J·cm-3)<supThen distill.
3. In the presence of phosphoric acid and diatomaceous earth catalysts, ethylene reacts directly with water to generate ethanol.
4. Use industrial ethanol as raw material, undergo dehydration treatment, and then perform distillation in a high-efficiency distillation tower. The resulting finished product can be filtered with a microporous membrane.
5. Select dry quicklime with high calcium oxide content and low iron, magnesium and sulfur impurities, crush it into small pieces with a diameter of 30mm, remove old ash, stones and slaked lime, and then mix it with 2 times the quality of industrial The ethanol is mixed and heated to reflux the ethanol. After about 18 hours, the dehydration is completed. Quickly steam out the ethanol, and remove a small amount of head liquid through rectification to obtain more than 99.5% reagent absolute ethanol. You can also pass 95% ethanol through a Na-type molecular sieve with a pore size of 4.2×10-9 for dehydration and removal of methanol, and then distill it. The molecular sieve can be baked at a high temperature of 400-500℃ for 3 hours, and then reused after activation.
6. Use ethylene glycol potassium acetate solution as the extraction agent, mix it with equal amounts of industrial ethanol, and then distill it in a high-efficiency distillation tower to obtain more than 99.7% anhydrous ethanol.
7. Using pentane or petroleum ether as an entrainer for distillation at 0.3~0.7MPa, more than 99.9% of absolute ethanol can be obtained.
8. Add the prepared anhydrous ethanol and an appropriate amount of metallic calcium into a container with a calcium chloride drying tube. After the metallic calcium fully absorbs water, the anhydrous solution that meets the gas chromatography standard can be obtained by distillation. Ethanol, ethanol content is greater than 99.95%. Industrial ethanol can also be used as raw material, and anhydrous ethanol that meets gas chromatography standards can be obtained through azeotropic distillation, gas phase preparative chromatography separation and purification.
9. When pure ethanol is produced, metal magnesium or metal sodium can be used to remove trace amounts of water in absolute ethanol. Ethanol with a large water content cannot be used directly to make absolute ethanol. Method for removing water from metallic magnesium: In a 1-liter round-bottomed flask equipped with a reflux condenser (with a calcium chloride drying tube on the top), put 2 to 3 grams of clean magnesium strips, 0.3 grams of iodine and 30 ml in order. 99.5 Pa ethanol, heat on a water bath until the iodine grains disappear completely (if there is no reaction, add a few more small grains of iodine). Continue heating, and after the magnesium is completely dissolved, add 500 ml of 99.5% ethanol. After refluxing for 1 hour, evaporate Ethanol, discard 10 ml of the first fraction, and collect the rest in a dry bottle for storage. The purity of this ethanol is >99.95%. Method for removing water with metallic sodium: The device is the same as above. Add 500 ml of 99.5% ethanol and 3.5 g of sodium to the bottle in sequence In, after the complete action, add a few grains of zeolite and 12.5 grams of ethyl succinate or 14 grams of diethyl phthalate, reflux for 2 hours, and then distill. Discard 10 ml of the previous fraction, and collect the rest in a dry Store in the bottle. Determination of trace moisture in ethanol: Add the benzene solution of aluminum ethoxide. If a large amount of white precipitate is formed, it indicates that the moisture content in ethanol exceeds 0.05%.
Purpose
1. Ethanol is an important organic solvent, widely used in medicine, coatings, sanitary products, cosmetics, oils and other methods, accounting for about 50% of the total ethanol consumption. Ethanol is an important basic chemical raw material, used to make acetaldehyde, ethylene, ethylamine, ethyl acetate, acetic acid, ethyl chloride, etc., and is derived from medicines, dyes, coatings, spices, synthetic rubber, detergents, etc. There are many intermediates for pesticides and other products, with more than 300 products. However, the use of ethanol as an intermediate for chemical products is gradually declining. Many products such as acetaldehyde, acetic acid, and ethyl ethanol no longer use ethanol as raw materials. Substitute other ingredients. 75% ethanol aqueous solution has strong bactericidal ability and is a commonly used disinfectant. Specially refined ethanol can also be used to make beverages. Similar to methanol, ethanol can be used as an energy source. Some countries have begun to use ethanol alone as automobile fuel or mixed with gasoline (more than 10%) to save gasoline.
2. Used as solvents for adhesives, nitrocellulose spray paints, varnishes, cosmetics, inks, paint strippers, etc., as well as raw materials for manufacturing pesticides, medicines, rubber, plastics, artificial fibers, detergents, etc., and also Can be used as antifreeze, fuel, disinfectant, etc. In the microelectronics industry, it is used as a dehydrating detergent and can be used in conjunction with degreasing agents.
3. Used as analytical reagents, such as solvents. Also used in the pharmaceutical industry.
4. Used in the electronics industry as dewatering detergent and degreaser ingredients. 5. Used to dissolve some water-insoluble electroplating organic additives, and also used as a reducing agent for hexavalent chromium in analytical chemistry.
5. Used in wine making industry, organic synthesis, disinfection and as solvent. [30]

 微信扫一扫打赏
微信扫一扫打赏

