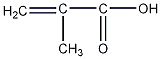
Structural formula
| Business number | 01PW |
|---|---|
| Molecular formula | C4H6O2 |
| Molecular weight | 86.09 |
| label |
2-methacrylic acid, A-methacrylic acid, α-methylsulfic acid, α-methacrylic acid, methacrylic acid, 2-Methylpropenoic acid, A-methyl-acrylic acid, α-methyl-abortion fatty acid, α-Methyl-acrylic acid, methyl propene acid (MAA), 2-Methyl-2-propenoic acid, intermediates, plexiglass, acidic solvent |
Numbering system
CAS number:79-41-4
MDL number:MFCD00002651
EINECS number:201-204-4
RTECS number:OZ2975000
BRN number:1719937
PubChem number:24883097
Physical property data
1. Properties: colorless crystal or transparent liquid with pungent odor. [1]
2. Melting point (℃): 16[2]
3. Boiling point (℃): 160 ~163[3]
4. Relative density (water = 1): 1.02[4]
5. Relative Vapor density (air=1): 2.97[5]
6. Saturated vapor pressure (kPa): 1.33 (60.6℃)[6]
7. Critical pressure (MPa): 4.7[7]
8. Octanol/water partition coefficient: 0.93[8]
9. Flash point (℃): 77 (OC) [9]
10. Ignition temperature (℃): 435[10]
11. Explosion upper limit (%): 8.7[11]
12. Explosion lower limit (%): 1.6[12]
13. Solubility: soluble in water, soluble in most organic solvents such as ethanol and ether. [13]
14. Refractive index at room temperature (n20): 1.4314
15. Liquid phase standard hot melt ( J·mol-1·K-1): 174.6
16. Solubility parameter (J·cm-3)0.5: 20.102
17. van der Waals area (cm2·mol-1): 7.650× 109
18. van der Waals volume (cm3·mol-1): 50.140
Toxicological data
1. Acute toxicity[14] LD50: 1600mg/kg (orally in mice); 500mg/kg (in rabbits through dermally) )
2. Irritation No data available
3. Subacute and chronic toxicity[15] Rat inhaled 4.5g/m3 (5h), 5h, 5 times, nose and eye irritation, weight loss, blood The urine test was normal, and the anatomy of internal organs was normal.
4. Mutagenicity[16] DNA adduct: E. coli 50μmol/L
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3. Non-biodegradability[17] In the air, when the concentration of hydroxyl radicals is 5.00×105pcs/cm3, the degradation half-life is 21h (theoretical).
Molecular structure data
1. Molar refractive index: 21.71
2. Molar volume (cm3/mol): 84.0
3. Isotonic specific volume (90.2K ): 198.2
4. Surface tension (dyne/cm): 30.9
5. Polarizability (10-24cm3): 8.60
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 83.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: Easy to polymerize into water-soluble polymers. It is flammable and may burn when exposed to high heat or open flames. It may decompose by heat to produce toxic gases. It can form explosive mixtures in air, with an explosion limit of 2.1%-12.5% (volume fraction).
2. This product has moderate toxicity and is highly irritating to skin and mucous membranes. Rat oral LD: 508400mg/kg, but no carcinogenic phenomenon has been found. The allowable limit concentration in the workplace is 100*10-6 (temporary working area). The production workshop requires good ventilation conditions, and operators must wear protective equipment, especially protective goggles. If in contact with skin, rinse with plenty of water.
3. Stability[18] Stable
4. Incompatible substances[19] Strong oxidants, amines, strong bases
5. Conditions to avoid contact [20] Light, heat, Ultraviolet rays, exposure to air
6. Polymerization hazards[21] Polymerization
Storage method
Storage Precautions[22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, amines, and alkalis, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
There are two main industrial production methods of methacrylic acid, namely the acetone cyanohydrin method and the isobutylene (tert-butyl alcohol) oxidation method.
1. Acetone cyanohydrin method Acetone and hydrocyanic acid react in the presence of an alkali catalyst to generate acetone cyanohydrin, which then reacts with concentrated sulfuric acid to generate methacrylamide sulfate, which is then hydrolyzed to generate methacrylamide sulfate. Acrylic. During production, it is required that acetone cyanohydrin and sulfuric acid do not contain moisture, otherwise acetone or α-hydroxymethyl isobutyrate will be produced and remain in the product, affecting product quality. The acetone cyanohydrin method is used to produce methacrylic acid. Each ton of product consumes 898kg of sodium cyanide (>87%), 1100kg of acetone (98.5%), and 4080kg of sulfuric acid (fuming).
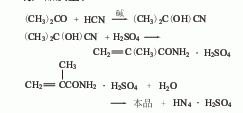
2. Isobutylene (tert-butyl alcohol ) Oxidation method: Isobutylene undergoes two-step oxidation, the first step generates methacrolein, the second step generates methacrylic acid, and then is distilled to obtain qualified products.
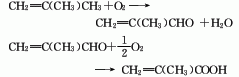
3. Methacrylonitrile water The solution method uses isobutylene as raw material and is obtained by ammonia oxygen and hydrolysis. 4. Isobutane oxidation method is used to obtain methacrolein through oxidation, and then it is obtained through oxidation.
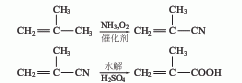
4. Isobutane oxidation method Methacrolein is obtained by oxidation, and then obtained by oxidation.
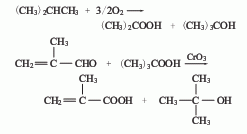
Purpose
1. Important organic chemical raw materials and polymer intermediates. Its most important derivative product, methyl methacrylate, produces organic glass that can be used in windows of aircraft and civil buildings, and can also be processed into buttons, solar filters, car light lenses, etc.; the coatings produced have superior suspension and rheology properties and durable properties; the resulting adhesives can be used for bonding metal, leather, plastics and building materials; methacrylate polymer emulsions are used as fabric finishing agents and antistatic agents. In addition, methacrylic acid can also be used as a raw material for synthetic rubber.
2. Organic chemical raw materials and polymer intermediates, used in the manufacture of methacrylates (ethyl methacrylate, glycidyl methacrylate, etc.) and organic glass. It is also used in the manufacture of thermosetting coatings, synthetic rubber, fabric treatment agents, leather treatment agents, ion exchange resins, insulation materials, antistatic agents, etc. It is a cross-linking monomer used in the manufacture of acrylic solvent-based and emulsion-based adhesives to improve the bonding strength and stability of the adhesive.
3. Used in organic synthesis and polymer preparation. [23]
�etc.) and plexiglass. It is also used in the manufacture of thermosetting coatings, synthetic rubber, fabric treatment agents, leather treatment agents, ion exchange resins, insulation materials, antistatic agents, etc. It is a cross-linking monomer used in the manufacture of acrylic solvent-based and emulsion-based adhesives to improve the bonding strength and stability of the adhesive.
3. Used in organic synthesis and polymer preparation. [23]

 微信扫一扫打赏
微信扫一扫打赏

