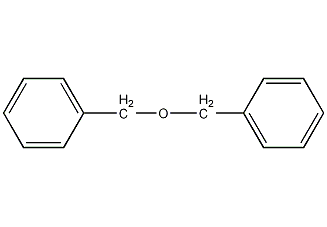
Structural formula
| Business number | 02NQ |
|---|---|
| Molecular formula | C14H14O |
| Molecular weight | 198.26 |
| label |
benzyl ether, Benzyl ether, plasticizer, Ether solvents, Mosquito repellent |
Numbering system
CAS number:103-50-4
MDL number:MFCD00004780
EINECS number:203-118-2
RTECS number:DQ6125000
BRN number:1911156
PubChem number:24846794
Physical property data
1. Properties: Colorless transparent liquid
2. Relative density (g/mL, 20/4℃): 1.0428
3. Relative density (g/mL, 15/15℃): 1.036
4. Relative vapor density (g/mL, air=1): 6.84
5. Melting point (ºC): 4
6. Boiling point (ºC, 101.3KPa): 298
7. Refractive index (n20ºC): 1.5618
8. Flash point (ºC, closed): 135
9. Vapor pressure (kPa, 200~201ºC): 2.67
10. Vapor pressure (kPa, 170 ºC): 2.13
11. Vapor pressure (kPa, 160 ºC): 1.47
12. Solubility: Insoluble in water, soluble in ethanol, ether, chloroform, acetone and other organic solvents.
13. Refractive index at room temperature (n25): 1.5592
14. Relative density (25℃, 4℃): 0.9782100
15. Liquid phase standard hot melt (J·mol-1·K-1): 342.3
Toxicological data
LD/LC50 amounts related to classification:
Oral LD50 4300mg/kg(mam)
2500mg/kg(rat)
Skin irritation mild 500mg/24h(rbt)
Eye irritation mild 500mg(rbt)
Main irritating effects:
On the skin: Irritating the skin and mucous membranes.
On eyes: Strong corrosive effect.
Sensitization: No known sensitizing effects.
Ecological data
General remarks
Ecotoxicological effects
Remarks: Toxic to fish
Water hazard class 2 (German regulations) (self-assessment via list) The Substances and their hazards to water.
Do not allow this product to come into contact with groundwater, waterways, or sewage systems, even in small amounts.
Even extremely small amounts of product seeping into the ground can pose a hazard to drinking water.
It is also poisonous to fish and plankton in water bodies.
Do not discharge materials into the surrounding environment without government permission.
Highly toxic to organic matter in water.
Molecular structure data
1.Molar refractive index: 62.03
2. Molar volume (cm3/mol): 189.3
3. Isotonic specific volume (90.2K): 475.6
4. Surface tension (dyne/cm): 39.8
5. Dielectric constant:
6. Dipole moment (10-24
sup>cm3):
7. Polarizability: 24.59
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 9.2
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 137
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxidizing agents. It is a flammable liquid. In case of fire, water, foam fire extinguishing agent, carbon dioxide, carbon tetrachloride, etc. can be used to extinguish the fire.
Chemical properties: Dibenzyl ether is unstable and can be gradually decomposed into benzaldehyde by moisture in the air at room temperature. When heated at 40~250℃, due to the oxidation of air, 80%~85% is converted into benzaldehyde, benzoic acid and benzyl benzoate. Heated with metallic sodium or sodium hydroxide, it produces toluene and benzoic acid. Diphenyl ether is heated on metallic sodium or reacts with sodium amide in liquid ammonia to rearrange to generate 1,2-diphenylethanol (C6H5CH2CHOHC6H5). In the presence of cobalt chloride, when reacting with butyl magnesium chloride (Grignard reagent), it decomposes into toluene and benzyl alcohol at room temperature.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
It is produced by the reaction between benzyl chloride and concentrated alkali. It is also a by-product during the production of benzyl alcohol.
Refining method: Dibenzyl ether often contains impurities such as benzaldehyde and toluene. Wash thoroughly with sodium hydroxide solution and sodium bisulfite solution to remove benzaldehyde. After drying over potassium carbonate, the toluene was fractionally distilled under reduced pressure. Distillation is carried out in a stream of nitrogen or carbon dioxide. It can also be refined by crystallization.
Purpose
It is used as a toughening agent for nitrocellulose and a solvent for preparing flavors. It can also be used as a moderate antioxidant, mainly used in the manufacture of sponge rubber products. Used as plasticizer for nitrocellulose and cellulose acetate, solvent for resin, rubber, wax, artificial musk, etc. A mixture of benzyl ether and zinc oxide is a repellent for mosquitoes, flies, gnats and fleas.

 微信扫一扫打赏
微信扫一扫打赏

