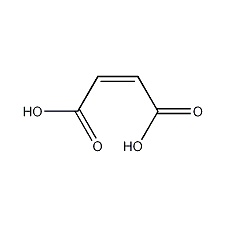
Structural formula
| Business number | 030P |
|---|---|
| Molecular formula | C4H4O4 |
| Molecular weight | 116.07 |
| label |
Maleic acid, (Z)-Butenedioic acid, Anhydrous malic acid, methacrylic acid, (Z)-Butenedioic acid, Toxilic acid, (Z)-1,2-Ethylene dicarboxylic acid, dyeing auxiliaries, preservative, acidic solvent |
Numbering system
CAS number:110-16-7
MDL number:MFCD00063177
EINECS number:203-742-5
RTECS number:OM9625000
BRN number:605762
PubChem number:OM9625000
Physical property data
1. Characteristics: Colorless crystal, with a special odor and a weak sour taste. Characteristic repulsive astringency.
2. Density (g/mL, 20℃): 1.59
3. Relative vapor density (g/mL, air=1): 4.0
4. Melting point (ºC): 130.5
5. Boiling point (ºC, normal pressure): 275
6. Relative density (20℃, 4℃): 1.590
7. Refractive index (n20D): 1.526
8. Flash point (ºC): 100
9. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -1466.2
10. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -679.4
11. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -1356.2
12. Crystal phase standard claimed heat (enthalpy) (kJ·mol-1): -789.4
13. Heat of combustion (KJ/ mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water ( Log value of the partition coefficient (octanol/water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/ V): Undetermined
19. Solubility: soluble in water, soluble in ethanol, acetone, glacial acetic acid, slightly soluble in benzene. Insoluble in benzene and chloroform.
Toxicological data
Citizenic acid is toxic and irritates the skin and mucous membranes.It can cause redness and swelling of the conjunctiva and cornea, as well as vision loss and even blindness. Skin contact with liquid acid can cause burns. The oral LD50 in rats is 708 mg/kg.
Ecological data
Non-biodegradability: Vapor state reacts with ozone and hydroxyl half-life 1.1 hours.
Bioconcentration or bioaccumulation:On land it can seep into groundwater and is biodegradable. It is biodegradable in water, but evaporation, adsorption and enrichment are not important return pathways.
Other harmful effects: Almost no adsorption to soil. Not easy to evaporate.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 23.76
2. Molar volume (cm3/mol): 77.4
3. Isotonic specific volume (90.2K): 222.0
4. Surface tension (dyne/cm): 67.6
5. Polarizability (10-24cm3): 9.42
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.3
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
p>
4. Number of rotatable chemical bonds: 2
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 74.6
p>
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 119
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Prohibit contact with alkalis, oxidants, and reducing agents.
2.Citic acid is toxic and irritates the skin and mucous membranes, causing redness and swelling of the conjunctiva and cornea, as well as Vision loss or even blindness. Skin contact with liquid acid can cause burns. The oral LD50 in rats is 708 mg/kg. During the operation, protective equipment must be worn, the equipment must be sealed to prevent leakage, and the site should have good ventilation conditions. Rinse with plenty of water after being contaminated with cisternic acid. In case of fire, use water or CO2 spray fire extinguisher. Do not use NaHCO3 fire extinguisher, because it can easily cause the rapid decomposition of cisternic acid.
3. Exist in tobacco leaves.
4. Strong irritation.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, reducing agents and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. The product is packaged in woven bags lined with plastic bags, with a net weight of 25kg per bag. Pay attention to moisture, fire and alkaline substances.
Synthesis method
1. Benzene oxidation method: After preheating, pure benzene is oxidized with air under the action of a vanadium series catalyst to generate maleic anhydride (hereinafter referred to as maleic anhydride). The reaction temperature is 360°C, and then absorbed and concentrated by water. , crystallize and dry to obtain the shun acid product.
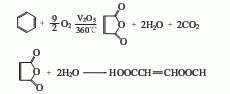
2. Butene (alkane) Oxidation method: Butene (alkane) is oxidized by air under the action of vanadium catalyst to generate maleic anhydride. The reaction temperature is 350-450°C. Then it is absorbed by water to generate maleic acid, which is then concentrated and dried to obtain the finished product.
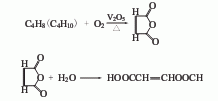
3. Other production methods: ( 1) When naphthalene or o-xylene is oxidized to produce phthalic anhydride, cisic acid is produced as a by-product; (2) Furfural oxidation method, this method is not used due to efficiency issues.
4.Under the catalysis of acetyl chloride, the water in malic acid is removed to form maleic anhydride, and then hydrolyzed into maleic anhydride Acid:
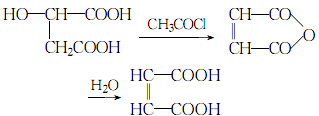
5 .Preparation method:
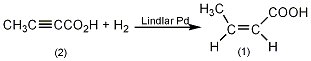 Add 100mg of Lindlar Pd catalyst into the hydrogenation unit. 2-butynoic acid (2) 2g (0.0024mol), 100mL ethanol, shake thoroughly to make 2-butynoic acid solution. Remove the air under reduced pressure, fill it with nitrogen, then extract the nitrogen, fill it with hydrogen, keep the hydrogen at factory pressure, and carry out hydrogenation under electromagnetic stirring. Stop hydrogenation when hydrogen gas absorption reaches 540 mL. Filter to remove catalyst. The solvent was evaporated under reduced pressure and cooled to below 15°C to precipitate white solid cis-butene-2-acid (1). Recrystallize with petroleum ether (40~60℃), mp15℃. [1]
Add 100mg of Lindlar Pd catalyst into the hydrogenation unit. 2-butynoic acid (2) 2g (0.0024mol), 100mL ethanol, shake thoroughly to make 2-butynoic acid solution. Remove the air under reduced pressure, fill it with nitrogen, then extract the nitrogen, fill it with hydrogen, keep the hydrogen at factory pressure, and carry out hydrogenation under electromagnetic stirring. Stop hydrogenation when hydrogen gas absorption reaches 540 mL. Filter to remove catalyst. The solvent was evaporated under reduced pressure and cooled to below 15°C to precipitate white solid cis-butene-2-acid (1). Recrystallize with petroleum ether (40~60℃), mp15℃. [1]
6. Preparation method:
Maleic anhydride (3): in a reaction bottle equipped with a stirrer and a reflux condenser (connected to a hydrogen chloride absorption device) , add 45g (0.33mol) of malic acid (2), slowly add 57mL (0.835mol) of acetyl chloride, and slowly heat the water bath with stirring to start the reaction. During the reaction, hydrogen chloride gas is generated and malic acid slowly dissolves. When the evolution of hydrogen chloride has basically stopped, continue heating in the water bath for 2 hours. Change to a distillation device, first steam out the low boiling point fraction, then collect the 195-200°C fraction, and solidify after cooling. Recrystallize with chloride to obtain 22g of maleic anhydride (3), with a yield of 67%. mp54℃. Maleic acid (1): Add 22g of the above compound (3) to 12mL of water and heat in a water bath. Then place it in a desiccator filled with concentrated sulfuric acid to remove the water. Finally, maleic acid (1) was obtained almost quantitatively, with mP143°C. The product is sufficiently pure for most experiments. It can be recrystallized from acetone-petroleum ether, mp144℃. [2]
Purpose
1. Preparation of pesticides Marathon, Dagenson, fumaric acid, unsaturated polyester resin, dyeing auxiliaries and oil preservatives, etc.
2.Used as H value indicator in nuclear magnetic resonance measurement. Used for organic synthesis, artificial resin preparation, and dyeing processing of wood, cotton, silk, etc. Fats and oils can be prevented from going rancid by adding 0.01%.
After chemical recrystallization, 22g of maleic anhydride (3) was obtained, with a yield of 67%. mp54℃. Maleic acid (1): Add 22g of the above compound (3) to 12mL of water and heat in a water bath. Then place it in a desiccator filled with concentrated sulfuric acid to remove the water. Finally, maleic acid (1) was obtained almost quantitatively, with mP143°C. The product is sufficiently pure for most experiments. It can be recrystallized from acetone-petroleum ether, mp144℃. [2]
Purpose
1. Preparation of pesticides Marathon, Dagenson, fumaric acid, unsaturated polyester resin, dyeing auxiliaries and oil preservatives, etc.
2.Used as H value indicator in nuclear magnetic resonance measurement. Used for organic synthesis, artificial resin preparation, and dyeing processing of wood, cotton, silk, etc. Fats and oils can be prevented from going rancid by adding 0.01%.

 微信扫一扫打赏
微信扫一扫打赏

