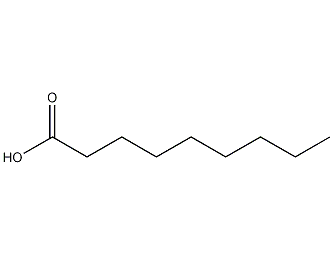
Structural formula
| Business number | 034X |
|---|---|
| Molecular formula | C9H18O2 |
| Molecular weight | 158.24 |
| label |
Fengyi oxalic acid, Geranic acid, Hydrangeaic acid, n-nonanoic acid, Grantrico, n-Nonanoic acid, Pelargonic acid, Nonoic acid, plasticizer, Desiccant, acidic solvent |
Numbering system
CAS number:112-05-0
MDL number:MFCD00004433
EINECS number:203-931-2
RTECS number:RA6650000
BRN number:1752351
PubChem number:24897735
Physical property data
1. Properties: Colorless oily liquid, industrial product is light yellow, with a slight special smell.
2. Density (g/mL, 20℃): 0.905
3. Relative density (20℃, 4℃): 0.9055
4. Melting point (ºC): 12.4
5. Boiling point (ºC, normal pressure): 255.6
6. Relative density (25℃, 4℃): 0.9017
7. Refractive index (n20D): 1.4319
8. Refractive index at room temperature (n20 ): 1.4322
9. Refractive index at room temperature (n25): 1.4302
10. Autoignition point or ignition temperature (ºC) : 405
11. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -5454.30
12. Saturated vapor pressure (kPa ,20 ºC): 0.04
13. The liquid phase standard claims heat (enthalpy) (kJ·mol-1): -659.73
14. Critical Temperature (ºC): 438.85
15. Critical pressure (KPa): 2.35
16. Solubility parameter (J·cm-3)0.5: 21.768
17. van der Waals area (cm2·mol-1): 1.463×10 10
18. van der Waals volume (cm3·mol-1): 104.800
19 . Solubility: Soluble in organic solvents such as ethanol, chloroform and ether, but insoluble in water.
20. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5537.98
21. Gas phase Standard claimed heat (enthalpy) (kJ·mol-1): -575.3
22. Liquid phase standard hot melt (J·mol-1 ·K-1): 358.7
23. Flash point (ºC): 100
Toxicological data
1. Acute toxicity: Mouse oral LD50: 5000mg/kg
Rat oral LD50: 5000mg/kg;
Rat skin LD5O: 2000mg/kg
Rabbit skin LD5O: 5000mg/kg
2. Irritation to skin and eyes : Rabbit skin 500mg/24H, moderate irritation. 100% guinea pig skin, severe irritation. 91mg has serious irritating effect on rabbit eyes.
Ecological data
Slightly harmful to water.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 45.43
2. Molar volume (cm3/mol): 171.7
3. Isotonic specific volume (90.2K): 412.0
4. Surface tension (dyne/cm): 33.1
5. Polarizability (10-24cm3): 17.96
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 3.5
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 7
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 37.3
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 99.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Quantity: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Corrosive. Mouse oral LD5015mg/kg. Intravenous injection of LD5024 mg/kg. Operators should wear protective equipment.
2. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves, and smoke.
3. Naturally found in bananas, beer, and bread.
4. Strongly irritating.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed. They should be stored separately from oxidants and acids, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials. 2. Plastic barrels for industrial products, 25kg per barrel. Analysis reagents are packed in glass bottles, 500g per bottle. Store and transport as general chemicals.
Synthesis method
1. The natural product of this substance (free or esterified state) exists in essential oils such as rose, geranium, iris, hops, and lavender. Industrially it is obtained by oxidation of 1-octene or oleic acid. 1. Oleic acid is oxidized by nitric acid or ozone.
![]()
2. Take 1-octene Using synthesis gas as raw materials, nonanal is obtained through carbonylation, and then nonanoic acid is obtained through air oxidation. During laboratory preparation, redistilled diethyl malonate can be added to sodium butoxide, heated to 80-90°C, and heptane bromide can be added to slowly reflux the reactant until the litmus becomes neutral. Then add potassium hydroxide solution and heat to reflux for 4-5 hours. After saponification is completed, steam distillation is performed until butanol no longer distills. Add concentrated hydrochloric acid to the residual liquid, reflux for 1 hour, cool and separate the oil layer and heat it until it reaches 180°C. When the release of carbon dioxide stops, the oil in the reactant is crude pelargonic acid. Distill under reduced pressure and collect the 140-142°C (1.6kPa) fraction to obtain the finished product pelargonic acid with a yield of about 70%.
![]()
3. Tobacco: OR, 26 , 44, 49; BU, 56; FC, 9, 40, 41; FC, BU, OR, 18; BU, 26; Synthesis: from oleic acid oxidized by nitric acid or ozone or from octene and synthesis gas as raw materials, hydroxyl It is converted into nonanal and obtained by air oxidation.
4. Preparation method:
In a 2L reaction bottle equipped with a stirrer, thermometer and reflux condenser, add 500mL anhydrous n-butanol and cut into small pieces in batches. Add 23g (1.0 mol) of metallic sodium into the lump and heat with stirring to complete the reaction of metallic sodium. Add 160g (1.0 mol) of diethyl malonate (2) at 70 to 80°C, and raise the temperature to 80 to 90°C. Remove the thermometer, install a dropping funnel, and add 182.5g (1.02mol) of 1-bromoheptane (3) dropwise to form sodium bromide precipitate. Control the dropping speed so that butanol does not flow out and slowly reflux. After the addition, continue the reflux reaction for 1 hour, and the reaction solution will become neutral. Transfer the reactants to a 3L reaction bottle, wash the reaction bottle with a small amount of water, find which one, transfer them together into a 3L reaction bottle, slowly add a solution of 140g potassium hydroxide dissolved in 140mL water, shake evenly, add zeolite, and load Reflux condenser and carefully heat to reflux for 5 hours to complete the hydrolysis (you can take a small amount of the reaction solution and add excess water to make it completely miscible). Steam distillation until no butanol distills. Slowly add 270 mL of concentrated hydrochloric acid to the residue, and heat to reflux for 1 hour. After cooling, separate the oil layer, add it to a 1L reaction bottle, install an air condenser, and heat the oil bath to 180°C until no carbon dioxide escapes, which takes about 2 hours. Transfer the residue into a distillation bottle, distill under reduced pressure, and collect the fraction at 140-142°C/1.6kPa to obtain 115g of product (1) with a yield of 73%. [1]
Purpose
1. Organic synthetic raw materials can be oxidized to produce azelaic acid, ammoniated and hydrogenated to produce nonylamine, and pressurized hydrogenation to produce nonanol. Pelargonic acid is also used in the production of pelargonic acid ester plasticizers and as a paint dryer. Pelargonic acid has a light aroma of fat and coconut. It is a permitted edible flavor according to my country’s GB2760-86 and is mainly used to prepare coconut and berry flavors.
2. Used in baked goods, meat products, and frozen dairy products.
In a bottle, distill under reduced pressure and collect the fraction at 140-142°C/1.6kPa to obtain 115g of product (1) with a yield of 73%. [1]
Purpose
1. Organic synthetic raw materials can be oxidized to produce azelaic acid, ammoniated and hydrogenated to produce nonylamine, and pressurized hydrogenation to produce nonanol. Pelargonic acid is also used in the production of pelargonic acid ester plasticizers and as a paint dryer. Pelargonic acid has a light aroma of fat and coconut. It is a permitted edible flavor according to my country’s GB2760-86 and is mainly used to prepare coconut and berry flavors.
2. Used in baked goods, meat products, and frozen dairy products.

 微信扫一扫打赏
微信扫一扫打赏

