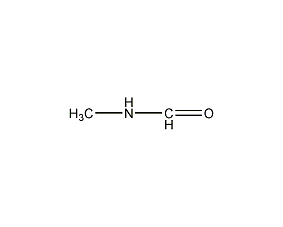
Structural formula
| Business number | 03GN |
|---|---|
| Molecular formula | C2H5NO |
| Molecular weight | 59.07 |
| label |
N-Formyl methylamine |
Numbering system
CAS number:123-39-7
MDL number:MFCD00003280
EINECS number:204-624-6
RTECS number:LQ3000000
BRN number:1098352
PubChem number:24896763
Physical property data
1. Properties: colorless and transparent liquid. It smells like ammonia.
2. Boiling point (ºC, 101.3kPa): 198~199
3. Melting point (ºC): -4
4. Relative density (g/ mL, 15/4ºC): 1.0075
5. Relative density (g/mL, 25/4ºC): 1.011
6. Refractive index (20ºC): 1.432
7. Refractive index (25ºC): 1.4300
8. Viscosity (mPa·s, 25ºC): 1.732
9. Viscosity (mPa·s, 35ºC): 1.468
10. Viscosity (mPa·s, 45ºC): 1.261
11. Flash point (ºC): 111
12. Conductivity (S/m ): 8×10-7
13. Body expansion coefficient (K-1, 25ºC): 0.0008691
14 . Solubility: Miscible with water and ethanol, slightly soluble in benzene, chloroform and ether.
Toxicological data
The oral LD50 in rats is 2700mg/kg. The LC50 of intravenous injection in mice is 4420mg/kg. The LC50 of mice intraperitoneally injected was 707mg/kg.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 15.09
2. Molar volume (cm3/mol): 67.6
3. Isotonic specific volume (90.2K ): 148.5
4. Surface tension (dyne/cm): 23.2
5. Polarizability (10-24cm3): 5.98
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 29.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 20
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Colorless transparent viscous oily liquid. It is soluble in water and can also dissolve inorganic salts. It is hygroscopic and easily decomposes in acidic or alkaline solutions. There is an ammonia smell.
Chemical properties and the interaction of hydrogen chloride can form��Two salts; generate HCONHCH3·HCl in non-polar solvents; generate (HCONHCH3)2·HCl in the absence of solvent. It has almost no interaction with metallic sodium at room temperature. Hydrolysis occurs under the influence of acid or alkali. The acidic hydrolysis rate is formamide>N-methylformamide>N,N-dimethylformamide. The rate of alkaline hydrolysis is formamide-N-methylformamide>N,N-dimethylformamide.
2. Exist in mainstream smoke.
Storage method
1.200kg iron drum packaging. Sealed storage to prevent leakage, rain, exposure, severe impact and friction. Keep away from fire and heat sources. Class 1 flammable liquid, Hazardous Regulation No. 61152.
Synthesis method
1. Methylamine method is produced by the reaction of methylamine and carbon monoxide.
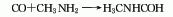
2. Methyl formate method Derived from the reaction of methyl formate and methylamine.

3. From ethyl formate and Derived from the reaction of methylamine. Add ethyl formate into the reactor, add methylamine aqueous solution under cooling, and reflux the reaction at 40°C. Then it was left for 3 days, and ethanol was recovered under reduced pressure to obtain crude product. The finished product is obtained by distillation under reduced pressure.
Refining method: The laboratory commonly uses alkali treatment for dehydration or the use of benzene-water azeotropic distillation to dehydrate and then refine for distillation. It can also be treated with molecular sieves and then refined by distillation.
4.Add calcium chloride to the mixture of formic acid and ethanol under cooling, and reflux for 12 to 13 hours to obtain a crude product. The crude product was neutralized with sodium carbonate, filtered and fractionated. The response is as follows:
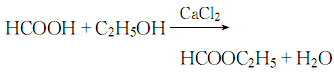
Under cooling, add the methylamine aqueous solution to the ethyl formate while stirring, control the temperature to about 40°C, and let it sit fully. The alcohol is recovered under reduced pressure, and the crude product is purified under reduced pressure to obtain the finished product. The response is as follows:
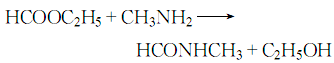
Purpose
1. Used as raw materials for organic synthesis, used in the synthesis of pesticides, insecticides, medicines, synthetic leather, artificial leather, etc.; and used as solvents for chemical fiber textiles, etc. In addition, N-methylformamide can be used to extract aromatic hydrocarbons from hydrocarbon mixtures.

 微信扫一扫打赏
微信扫一扫打赏

