
Structural formula
| Business number | 035U |
|---|---|
| Molecular formula | C10H22O2 |
| Molecular weight | 174.28 |
| label |
1,10-Dihydroxydecane, Decanediol, Decamethine glycol, Decanediol, Decamethylene glycol, Decylene glycol, Decamethylenediol, Decamethylene glycol, linear compound |
Numbering system
CAS number:112-47-0
MDL number:MFCD00004749
EINECS number:203-975-2
RTECS number:HD8433713
BRN number:1698975
PubChem number:24893376
Physical property data
1. Properties: White needle-like crystals.
2. Relative density (25℃, 4℃): 0.8504130
3. Relative density (20℃, 4℃): 0.88380
4. Melting point (ºC): 73
5. Boiling point (ºC, normal pressure): 175.515
6. Boiling point (ºC, 2.67KPa): 192
7. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -6385.8
8. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -693.5
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 70ºC): Undetermined
12 . Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
p>
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in alcohol and hot ether. Almost insoluble in cold water and petroleum ether.
Toxicological data
1. Acute toxicity: mouse intraperitoneal LD: >500mg/kg
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 51.44
2. Molar volume (cm3/mol): 188.6
3. Isotonic specific volume (90.2K): 463.4
4. Surface tension (dyne/cm): 36.4
5. Polarization.�� (10-24cm3): 20.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 9
5. Number of tautomers: none
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 64.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxides.
Storage method
Stored sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep away from sources of fire. Store away from oxidizing agents.
Synthesis method
1. Obtained from sebacic acid through esterification and reduction. 1. Esterification: Add sebacic acid, ethanol, benzene and p-toluenesulfonic acid into a reaction pot equipped with a water separator, heat to reflux, and bring water for about 4-5 hours until no water separates. Cool and filter to obtain decane. Crude diethyl diacid. The yield is 85%. 2. For reduction, first put diethyl sebacate and absolute ethanol into the reaction pot, then gradually add the metal sodium while stirring and cooling, and heat and reflux until the metal sodium is completely dissolved and no residue remains. Carry out steam distillation to recover ethanol, then add water to dilute, cool, and the precipitated crude product is recrystallized with benzene to obtain the finished product, with a yield of 54%.
2. Preparation method:
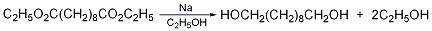
In a dry reaction bottle equipped with a stirrer, ventilation tube, reflux condenser (installed with a calcium chloride drying tube) and dropping funnel, add 65g of diethyl sebacate (2) ( 0.25mol), 800mL of absolute ethanol. Cool in ice water bath with stirring. Add 70g (3.0mol) of fresh sodium metal cut into pieces at one time, the reaction proceeds vigorously, and hydrogen gas escapes. After the reaction is stable, heat the water bath until the reaction of metallic sodium is complete. After cooling slightly, add 300 mL of water and evaporate the ethanol. Finally, distillation under reduced pressure was performed to remove residual ethanol. Add 600mL of hot water and let it cool. Separate the oil (retain the water layer) and solidify after cooling. The solid was washed with a small amount of water and dried under vacuum. The solid material was extracted with benzene four times (250 mL × 4), the benzene layers were combined, and decolorized with activated carbon. Concentrate to about 60 mL, add 200 mL of ethanol, filter, concentrate to about 60 mL, add an equal volume of benzene, and heat to dissolve. Cool and solid precipitates. After suction filtration and washing with diethyl ether, 32g of 1,10-decanediol (1) was obtained, mp 72~74°C, yield 73%. [1]
Purpose
Pharmaceutical intermediates.

 微信扫一扫打赏
微信扫一扫打赏

