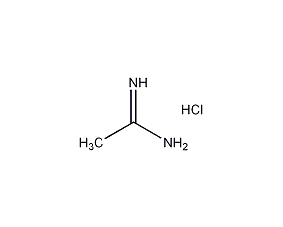
Structural formula
| Business number | 03J7 |
|---|---|
| Molecular formula | C2H7ClN2 |
| Molecular weight | 94.54 |
| label |
Acetamidine hydrochloride, Ethanimidamide hydrochloride, Acediamine hydrochloride, a-Amino-a-imioethane hydrochloride |
Numbering system
CAS number:124-42-5
MDL number:MFCD00013016
EINECS number:204-700-9
RTECS number:None
BRN number:3591762
PubChem ID:None
Physical property data
1. Properties: White to slightly yellow long prismatic crystals
2. Melting point (℃): 164~166
3. Solubility: Easily soluble in water, Soluble in ethanol and methanol, almost insoluble in ether and acetone.
Toxicological data
None
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area: 49.9
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 31
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. When encountering alkali solution, acetamidine is immediately released, and when slightly heated, it decomposes into ammonia and acetic acid.
2. It is irritating to eyes and skin. Protective clothing should be worn when using in large quantities. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
Storage method
Storage: Store in a cool and dry place
This product is packed in polyethylene film-lined bags, each bag is 25kg.
Synthesis method
Brief description of production method: It is obtained by addition and amination of acetonitrile, hydrochloric acid and methanol. Circulate methanol in the turbulent ball tower with a pressure of less than 26.6kPa. Dry hydrogen chloride is introduced under cooling to prepare a hydrogen chloride methanol solution with a concentration of 45%. Then put it into the reaction tank, stir and cool to 10-5°C, add acetonitrile dropwise, and keep it at 15-20°C for 6 hours to obtain iminomethyl ethyl ether hydrochloride. Put it into the pre-prepared 13% ammonia methanol solution, ammonolyze it at 0-40°C, keep the pH value at 7-8, stir for 2-3 hours, cool down to below 30°C, centrifuge and filter out the ammonium chloride. Methanol is recovered from the filtrate, and the finished product is obtained by cooling and filtering. Weight input ratio: acetonitrile: hydrogen chloride methanol solution (45%): ammonia: methanol = 1:3:1.05:4.3. The yield is 82%-85%.
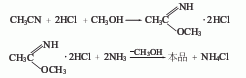
Purpose
Used in the synthesis of imidazole, pyrimidine, triazine antibiotics, and vitamin B1.

 微信扫一扫打赏
微信扫一扫打赏

