Background and overview[1]
2,2,5,7,8-pentamethylchroman-6-sulfonyl chloride can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
2,2,5,7,8-pentamethylchroman-6-sulfonyl chloride is prepared as follows:
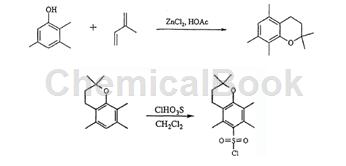
1) Mix 2,3,5-trimethylphenol (50.03g, 0.367 mol), isoprene (25.09g, 0.368 mol) and molten zinc chloride (5.94g, 0.044 mol) with anhydrous Acetic acid (47ml) was stirred at room temperature for 14 hours. The cloudy red mixture is then gradually heated and becomes clear. After refluxing, the reaction mixture turned black. After refluxing for 8 hours, it was cooled to room temperature. The reaction mixture was poured into 250 ml of water and a black oil separated. The water was extracted with pentane (3 x 200 ml), the combined organic phases were washed with Claisen’s base (2 x 150 ml), water (3 x 250 ml) and brine (2 x 200 ml), dried over CaCl2 and evaporated under reduced pressure into brown oil. The crude product was distilled at 0.48 mBar to obtain the product as a light yellow liquid (36.90 g, 49% yield); m/z 193. b.p.82-96°C (0.48mBar); Purity >95% (GC). The product isolated as a pale yellow liquid, which solidified on cooling, with a yield of 49%.
1HNMR (CDCl3, 400MHz): δ=1.30 (6H, S, 2xCH3), 1.78 (2H, t, J=7Hz, CH2), 2.07 (3H, s, CH3), 2.15 (3H, s, CH3 ), 2.19 (3H, s, CH3), 2.59 (2H, t, J=7Hz, CH2), 6.54 (1H, s, aromatic H).
13CNMR (CDCl3, 400MHz): δ=11.42 (CH3), 18.91 (CH3), 19.84 (CH3), 20.49 (CH2), 26.97 (2xCH3), 32.79 (CH2), 73.10 (C (CH3)2) , 116.67 (Ar-C), 122.03 (Ar-C), 122.29 (Ar-C), 133.44 (Ar-C, 134.70 (Ar-C), 151.72 (Ar-C).
2) Add 2,2,5,7,8-pentamethylchroman (3.39g, 16.6mrαol) in 30ml dichloro To the solution in methane was added a solution of chlorosulfonic acid (3.98 g, 34.2 mmol) in 30 ml of dichloromethane for 1 min. The mixture was stirred at -8°C for 15 minutes and at room temperature for 2.5 hours. The reaction mixture was carefully shaken several times with 50 ml of methylene chloride and 100 ml of ice and the phases were separated. The crude product contained approximately 16% of starting material as judged by 1H NMR. When hot pentane was added to the crude product, a dark oil formed, which was removed by decantation. The product 2,2,5,7,8-pentamethylchroman-6-sulfonyl chloride was then isolated by crystallization from pentane as a light brown powder (2.80 g, 9.3 mmol). Yield 56%.
1HNMR (CDCl3): δ1.34 (s, 6H), 1.85 (t, J=7.0Hz, 2H), 2.14 (s, 3H), 2.60 (s, 3H), 2.62 (s, 3H), 2.68 (t, J=7.0Hz, 2H).
Main reference materials
[1] (WO2000012541) CYTOTOXIC PEPTIDES MODIFIED BY BULKY OR LIPOPHILIC MOIETIES

 微信扫一扫打赏
微信扫一扫打赏

