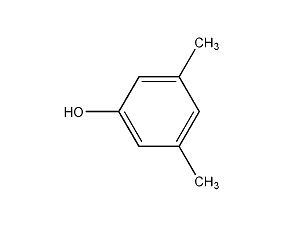
Structural formula
| Business number | 02XQ |
|---|---|
| Molecular formula | C8H10O |
| Molecular weight | 122.16 |
| label |
1-hydroxy-3,5-dimethylbenzene, 1,3-dimethyl-5-hydroxybenzene, 3,5-dimethylphenol, 3,5-Dimethylphenol, 5-Hydroxy-m-xylene, 1-Hydroxy-3,5-dimethylbenzene, sym-m-Xylenol, Steel rolling cold rolling oil additives, phenolic solvents, aromatic compounds |
Numbering system
CAS number:108-68-9
MDL number:MFCD00002307
EINECS number:203-606-5
RTECS number:ZE6475000
BRN number:774117
PubChem number:24862066
Physical property data
1. Characteristics: white crystal.
2. Boiling point (ºC, 101.3kPa): 222
3. Boiling point (ºC, 13.3kPa): 155
4. Boiling point (ºC, 2.67 kPa): 117.5
5. Melting point (ºC): 63.4
6. Relative density (g/mL, 25/25ºC, solid): 1.115
7. Relative density (g/mL, 80/4ºC): 0.968
8. Flash point (ºC): 109
9. Kinematic viscosity (m2/s, 80ºC): 0.0250×10-4
10. Kinematic viscosity (m2/s, 120ºC): 0.01075×10 -4
11. Kinematic viscosity (m2/s, 160ºC): 0.00635×10-4
12. Heat of evaporation (KJ/mol): 49.34
13. Heat of generation (KJ/mol): 244.64
14. Heat of combustion (KJ/mol) : 4335.72
15. Critical temperature (ºC): 442.4
16. Vapor pressure (kPa, 62ºC): 0.133
17. Solubility: hardly soluble In water, miscible with ethanol, chloroform, ether, benzene, etc. Soluble in sodium hydroxide aqueous solution.
18. Refractive index at room temperature (n25): 1.513025
19. Relative density (25℃, 4℃ ): 0.96880
20. Relative density (20℃, 4℃): 0.970574
21. Crystal phase Phase standard combustion heat (enthalpy) (kJ·mol-1): -4332.8
22. Crystal phase standard claim heat (enthalpy) (kJ·mol-1 ): -244.4
23. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4415.6
24. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -161.5
25. Gas phase standard��(J·mol-1·K-1): 391.80
26. Gas phase standard free energy of formation (kJ·mol– 1): -39.3
27. Gas phase standard hot melt (J·mol-1·K-1): 152.70
Toxicological data
1. Skin/eye irritation: Standard Draize test: rabbit, skin contact: 250μg, severity of reaction: severe.
2, acute toxicity: Rat LD50: 608mg/kg; Rats inhaled LC:> 4mg/m 3 ;
mice Oral LD50: 900mg/kg; Mouse oral LD50: 477g/kg;
Mouse abdominal LD50: 146mg/kg; Rabbit oral LD50: 1313mg/kg;
3. Chronic toxicity/carcinogenicity: Rat skin contact TDLo: 4000mg/kg/20W-I;
4. Steam is irritating to the eyes and respiratory mucosa.
Ecological data
BOD5 (five-day biological oxygen demand): 0.82 ThOD: 2.619 This substance is harmful to the environment. Special attention should be paid to the pollution of air, water environment and water sources.
Molecular structure data
1. Molar refractive index: 37.78
2. Molar volume (cm3/mol): 120.4
3. Isotonic specific volume (90.2K ): 297.5
4. Surface tension (dyne/cm): 37.2
5. Dielectric constant:
6. Dipole moment (10-30C·m): 5.57
7. Polarizability: 14.97
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 10
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 80.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxides. Corrosive and toxic. Can burn when exposed to open fire.
2. Exist in oriental tobacco leaves and mainstream smoke.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Distillate mixed xylenol, collect the 218-222°C fraction, and obtain industrial grade 3,5-xylenol through cooling, crystallization, and centrifugal separation. Then recrystallize with toluene to obtain a finished product with a purity of 98%. M-xylene is sulfonated with concentrated sulfuric acid at 185°C, neutralized at 60-90°C, alkali fused at about 350°C, acidified with sulfuric acid, and finally distilled under reduced pressure to obtain 3,5-xylenol with a yield of 65 %.
2. Tobacco: OR, 26.
Purpose
Used in the production of pesticides, rubber accelerators, antioxidants, drugs, spices, phenolic resins, etc. It is also used as an additive for steel rolling and cold rolling oil to extend the service life of cold rolling oil.

 微信扫一扫打赏
微信扫一扫打赏

