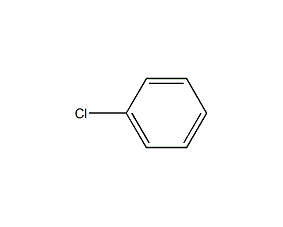
Structural formula
| Business number | 02Y7 |
|---|---|
| Molecular formula | C6H5Cl |
| Molecular weight | 112.56 |
| label |
Monochlorobenzene, phenyl chloride, Benzene chloride Monochlorobenzene, Pesticide intermediates; aromatic halogen derivatives |
Numbering system
CAS number:108-90-7
MDL number:MFCD00000530
EINECS number:203-628-5
RTECS number:CZ0175000
BRN number:605632
PubChem number:24857162
Physical property data
1. Properties: colorless and transparent liquid with an unpleasant bitter almond smell. [1]
2. Melting point (℃): -45.2[2]
3. Boiling point (℃): 131.7[3]
4. Relative density (water = 1): 1.11[4]
5. Relative vapor Density (air=1): 3.88[5]
6. Saturated vapor pressure (kPa): 1.17 (20℃)[6]
7. Heat of combustion (kJ/mol): -3100[7]
8. Critical temperature (℃): 359.2[8]
9. Critical pressure (MPa): 4.52[9]
10. Octanol/water partition coefficient: 2.18~2.89[10]
11. Flash point (℃): 29[11]
12. Ignition temperature (℃) : 638[12]
13. Explosion upper limit (%): 11[13]
14. Explosion lower limit (%) %): 1.3[14]
15. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol, ether, chloroform, carbon disulfide, and benzene. [15]
16. Viscosity (mPa·s, 20ºC): 0.799
17. Heat of evaporation (KJ/mol, b.p.): 36.57
18. Heat of fusion (KJ/mol): 9.56
19. Heat of formation (KJ/mol, 25ºC, liquid): -10.68
20. Combustion Heat (KJ/mol, 25ºC, liquid): 3110.96
21. Specific heat capacity (KJ/(kg·K), 20ºC, liquid, constant pressure): 1.29
22. Conductance Rate (S/m, 25ºC): 7×10-11
23. Solubility (%, water, 30ºC): 0.0488
24. Body Expansion coefficient (K-1, 20ºC): 0.00098
25. Relative density (25℃, 4℃): 1.1012
26. Refractive index at room temperature (n25): 1.5223
27. Critical density (g·cm-3): 0.365
28. Critical Volume (cm3·mol-1): 308
29. Critical compression factor: 0.265
30. Eccentricity factor : 0.251
31. Solubility parameter (J·cm-3)0.5: 19.264
32. van der Waals Area (cm2·mol-1): 7.140×109
33. van der Waals volume (cm 3·mol-1): 57.840
34. Gas phase standard claims heat (enthalpy) (kJ·mol-1): 52.0
35. Gas phase standard entropy (J·mol-1·K-1): 314.14
36. Gas phase standard free energy of formation (kJ·mol-1): 99.3
37. Gas phase standard hot melt (J·mol-1· K-1): 97.99
38. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): 11.0
39. Liquid phase standard entropy (J·mol-1·K-1): 209.2
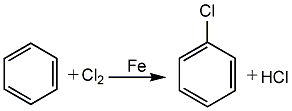
40. Liquid phase standard generation is free Energy (kJ·mol-1</su��, this was the first method of industrial production in the United Kingdom in 1909 and has been used to this day. There are two types of chlorination processes: gas phase method and liquid phase method. The gas phase method has a reaction temperature of 400 to 500°C and the cost is higher than the liquid phase method, so it has been eliminated; the liquid phase method has two types: batch method and continuous method. (1) In the intermittent method, put dry benzene into the chlorination reactor, and then add iron filings equivalent to 1% of the benzene amount as a catalyst. The chlorine gas should be added at a rate that can maintain the reaction temperature at 40 to 60°C. High is conducive to the production of polychlorinated benzene. Bubble chlorine gas into the benzene until the relative density of the liquid reaches 1.280 (15°C). The hydrogen chloride released by the reaction is washed with benzene or chlorobenzene to remove organic droplets, and then absorbed with water to obtain hydrochloric acid. The chlorinated material is neutralized with 10% sodium hydroxide, dried and distilled to obtain the following fractions (based on 100% chlorinated material): benzene and water (3%), benzene and chlorobenzene (10%), this The second fraction is returned to the system; chlorobenzene (75%) is used as the product; chlorobenzene and dichlorobenzene (10%), high boilers (2%), this second fraction is used to separate o- and p-dichlorobenzene. The composition of the chlorinated product depends on the chlorination temperature, chlorination rate, chlorination depth and the catalyst used. Generally, the composition of chlorinated products is 80% chlorobenzene, 15% p-dichlorobenzene, and 5% o-dichlorobenzene and polychlorinated benzene. (2) Continuous chlorination is carried out at the boiling temperature of benzene. The chlorinator is equipped with a catalyst (iron filings or anhydrous ferric chloride), and the heat of reaction is taken out by the gasification of benzene and a small amount of chlorobenzene. The dried benzene is measured by a rotor flowmeter and then added to the bottom of the chlorinator, where it reacts with the measured dry chlorine gas downstream into the chlorinator. The HCl and part of the benzene and chlorobenzene vapors produced by the reaction are condensed in the graphite condenser, and then sprayed and absorbed with crude chlorobenzene in the absorption tower. When the benzene content of the absorption liquid reaches 32% to 36%, it is mixed with acidic chlorinated liquid for the neutralization process, and the gas is absorbed into 31% of by-product hydrochloric acid. The acidic chlorinated liquid flowing out of the chlorinator is washed with water, neutralized with alkali solution to remove residual acid and ferric chloride, and then passed through a salt dryer, preheated to a certain temperature and then added to the crude distillation tower, and benzene is taken out from the top of the tower. . The crude chlorobenzene in the tower still is continuously added to the distillation tower, and chlorobenzene is obtained from the top of the tower. The residual liquid in the tower still is intermittently released, and the dichlorobenzene in it is recovered.
2. Use industrial chlorobenzene as The raw material is distilled to collect fractions between 130 and 133°C; the distillation residue mainly contains three isomers of dichlorobenzene and 1,2,4-trichlorobenzene, which can be used to extract these substances. To prepare chromatographically pure chlorobenzene, nitrogen can be used as the carrier gas, and industrial chlorobenzene can be injected into a preparative gas chromatograph equipped with a DBS* stationary phase or SE30/red diatomite carrier stationary phase column, and its main peak components can be collected through separation. , then put it into a glass ampoule and seal it. DBS*: A gas chromatography stationary phase based on anionic surfactants.
Purpose
1. Used as intermediates for dyes, medicines, pesticides, and organic synthesis. It is also used to prepare solvents and rubber additives, paints, quick-drying inks and dry cleaning agents.
2. Used as a solvent for nitrocellulose spray paints, coatings and varnishes. Industrially used as raw materials for the manufacture of aniline, phenol, picric acid, dyes, medicines, spices, pesticides, etc.
3. Used as solvent, gas chromatography reference material, used in organic synthesis, and also used in electronic industry products and raw material inspection.
4. As an important raw material for organic synthesis. [29]

 微信扫一扫打赏
微信扫一扫打赏

