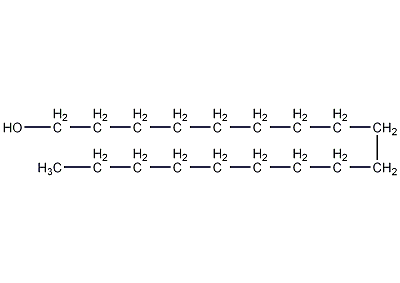
Structural formula
| Business number | 036X |
|---|---|
| Molecular formula | C18H38O |
| Molecular weight | 270.49 |
| label |
Octadeca fatty alcohol, 1-Hydroxyoctadecane, Fatty wax alcohol, 1-Octadecanol, stearyl alcohol, stearyl alcohol, 1-Hydroxyoctadecane, Stearyl alcohol, Octadecyl alcohol, 1-Octadecanol, Stenol, emulsifier, Dispersant, defoamer, polish, lubricants, couplers, rice field insulation agent, reservoir covering agent, alcohol solvent |
Numbering system
CAS number:112-92-5
MDL number:MFCD00002823
EINECS number:204-017-6
RTECS number:RG2010000
BRN number:1362907
PubChem number:24886710
Physical property data
1. Characteristics: White waxy leaf crystals at room temperature, with fragrance.
2. Density (g/cm3): 0.812
3. Relative vapor density (g/mL, air=1): 9.3
3. p>
4. Melting point (ºC): 57.5
5. Boiling point (ºC): 210.5
6. Refractive index (20ºC): 1.45
7. Flash point (ºC): 185
8. Autoignition point or ignition temperature (ºC): 247.8
9. Vapor pressure (mmHg, 38ºC): <0.01
10. Solubility: Insoluble in water, soluble in ethanol and ether, slightly soluble in benzene, chloroform and acetone.
11. Refractive index at room temperature (n25): 1.438860
12. Relative density (25℃, 4℃ ): 0.812360
13 Boiling point (ºC): 20310
Toxicological data
1. Irritation: Rabbit transdermal: 500mg/24 hours, mild irritation. Rabbit eye: 100mg/24 hours, mild irritation
2. Acute toxicity: Mouse oral LD5O: 20000mg/kg
3. It is slightly toxic. Low vapor pressure, non-irritating and harmful to human skin.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water pollution.
Molecular structure data
1. Molar refractive index: 86.97
2. Molar volume (cm3/mol): 323.1
3. Isotonic specific volume (90.2K ): 765.0
4. Surface tension (dyne/cm): 31.4
5. Polarizability (10-24cm3): 34.47
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 16
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 19
8. Surface charge: 0
9. Complexity: 145
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxides and acids. It undergoes sulfonation with concentrated sulfuric acid and does not react chemically with alkali.
2. Exist in tobacco leaves and smoke.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. 2. They should be stored separately from oxidants and acids, and avoid mixed storage. 3. Equip with corresponding varieties and quantities of fire-fighting equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. It can be obtained by hydrolysis of pilot whale oil, hydrogenation of stearic acid under the catalysis of copper chromate, or reduction of ethyl stearate with saturated ethanol. The heptadecene fraction can also be obtained by controlling the polymerization reaction of ethylene under the action of alkyl aluminum, and then octadecyl alcohol can be obtained through oxo synthesis.
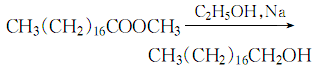
To prepare chromatographically pure stearyl alcohol, nitrogen can be used as the carrier gas and fixed on a SE30/white silanized diatomite carrier. Preparation of the phase column: Inject the crude product into the gas chromatograph, separate and collect the main peak components, and then seal and store it.
Purpose
1. Stearyl alcohol can be used instead of cetyl alcohol, such as dispersing agent in pharmaceuticals, creams and emulsifiers in cosmetics, and also used as rice field heat preservation agent, reservoir covering agent, defoaming agent, etc. Polishing agents, lubricants, etc. Stearyl alcohol can be used in the production of flat plus, resin and synthetic rubber, color film couplers, etc. Stearyl alcohol and β-(3,5-di-tert-butyl-4-hydroxyphenyl) methyl propionate are reacted at 130°C for 2-3 hours using sodium methoxide as the catalyst and dimethyl sulfoxide as the solvent. Oxygen 1076 (2082-79-3). This is an excellent non-polluting non-toxic antioxidant, widely used in petroleum products such as polyolefin, polyamide, polyester, polyvinyl chloride, polystyrene, ABS resin and various rubbers.
2.Can be used instead of cetyl alcohol as an additive for oil-based fracturing fluids. Stearyl alcohol can be used in the production of flat plus, resin and synthetic rubber, color film couplers, etc.
3.Used as chromatographic reagents. Also used as surfactant and lubricant. Used in organic synthesis.
4.In addition, it can also be used to manufacture defoaming agents, flotation agents, softeners, rice field insulation agents, water surface covering agents, Raw materials for medical ointments and couplers for color films.

 微信扫一扫打赏
微信扫一扫打赏

