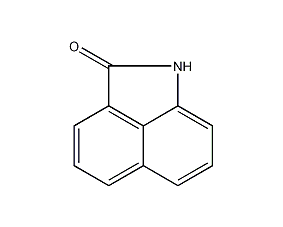
Structural formula
| Business number | 03MA |
|---|---|
| Molecular formula | C11H7NO |
| Molecular weight | 169.18 |
| label |
naphthalimide, 1,2-Dihydrobenzo[cd]indol-2-one, 1,8-Naphtholactam, aromatic compounds |
Numbering system
CAS number:130-00-7
MDL number:MFCD00009748
EINECS number:204-973-4
RTECS number:DE3202000
BRN number:None
PubChem number:24860382
Physical property data
1. Properties: fine needle crystals
2. Melting point (℃): 181
3. Solubility: soluble in boiling water, insoluble in ether.
Toxicological data
1. Acute toxicity: unknown mammalian LD5O: 2700mg/kg
Mouse oral LD5O: 1gm/kg
2. Other multiple dose toxicity: rat oral TDLO: 4200mg/kg/14D-I
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 50.52
2. Molar volume (cm3/mol): 127.4
3. Isotonic specific volume (90.2K ): 350.7
4. Surface tension (dyne/cm): 57.3
5. Polarizability (10-24cm3): 20.02
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 29.1
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 238
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
Derived from rearrangement of 1,8-naphthalenedicarboximide. Add water, sodium hydroxide solution and caustic potassium solution to the reaction pot, add sodium hypochlorite and diffusing agent NNO while stirring, add 1,8-naphthalenedicarboximide at 30°C, and keep it at 28-30°C for 2.5 hours. Maintain an excess of sodium hypochlorite during the reaction. After the heat preservation is completed, use sodium bisulfite to eliminate excess sodium hypochlorite, and use hydrochloric acid to adjust to 9.5-10. Filter out the unreacted naphthalene diimide, and precipitate the filtrate with hydrochloric acid until the pH is 2-3 at a temperature below 40°C. Heat to 80-85°C, keep warm for half an hour, and close the loop. Cool to 60°C, filter and wash to obtain 1,8-naphthalene lactimide.
Purpose
Dye intermediates. Used to synthesize perylene dyes and pigments, such as reduced gray BG, reduced brilliant orange 3RK, soluble anthracene brilliant orange IRK, etc.

 微信扫一扫打赏
微信扫一扫打赏

