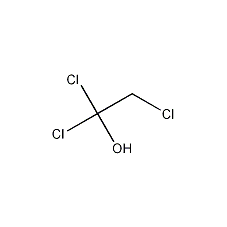
Structural formula
| Business number | 037Q |
|---|---|
| Molecular formula | C2H3Cl3O |
| Molecular weight | 149.40 |
| label |
trichloroethanol, Trichloroethanol, aliphatic compounds |
Numbering system
CAS number:115-20-8
MDL number:MFCD00004677
EINECS number:204-071-0
RTECS number:KM3850000
BRN number:1697495
PubChem number:24900284
Physical property data
1. Properties: Colorless liquid, easily hygroscopic, with an ether smell.
2. Density (g/mL, 20℃): 1.57
3. Relative vapor density (g/mL, air=1): 5.6
4. Melting point (ºC): 19
5. Boiling point (ºC, normal pressure): 151737
6. Boiling point (ºC, 16.67KPa) :94-97
7. Refractive index (n20/D): 1.4861
8. Flash point (ºC): >110
9. Specific rotation (ºC): Undetermined
10. Autoignition point or ignition temperature (ºC): 151
11. Vapor pressure (mmHg, 38ºC ): Undetermined
12. Saturated vapor pressure (kPa, 28ºC): 5.32
13. Heat of combustion (KJ/mol): Undetermined
14 . Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19 . Solubility: Miscible with alcohol and ether, soluble in water.
Toxicological data
1. Acute toxicity: Rat oral LDLO: 500mg/kg
Mouse oral LDLO: 500ppm/4H
Rat intraperitoneal LDLO: 300mg/kg
Rabbit meter veins LDLO: 50mg/kg
Rabbit through the rectal LDLO: 500mg/kg
2. Other dose toxicity: Rat meter TDLO: 4200mg: 4200mg /kg/14D-I
Rat oral TDLO: 14400mg/kg/90D-I
3. Mutagenicity: Aspergillus mutation: 7750ug/plate
Aspergillus sex chromosome deletion and non-disjunction: 10240umol/L
Human lymphocyte sister chromatid exchange: 178gm/L
Ecological data
Mildly hazardous to water.
Molecular structure�Cool. Slowly add a solution of 1.3g (0.03mol) sodium borohydride dissolved in 20mL cold water dropwise. The reaction is exothermic, and the temperature of the reaction solution is controlled so that the solution becomes acidic. Extract with ether, wash with a small amount of water, and dry over anhydrous magnesium sulfate. Steam off the ether, then heat and distill, collect the fraction at 151-153°C to obtain 9.8g of 2,2,2-trichloroethanol (1), with a yield of 65%. [3]
4. Preparation method:
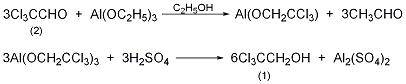
In a reaction flask equipped with a stirrer and a high-efficiency fractionation column, add 250g (1.7mol) of anhydrous trichloroacetaldehyde (2), 700mL of anhydrous ethanol, and 75g of aluminum ethoxide (0.46mol), and heat to boiling , slowly fractionate the acetaldehyde generated, and the reaction ends in about 24 hours. The ethanol is recovered to dryness, and finally the ethanol is distilled under reduced pressure. Cool, slowly add 20% sulfuric acid, and steam distill. Separate the oil layer. The aqueous layer was saturated with sodium sulfate and extracted three times with diethyl ether. The ether layer and oil layer were combined and dried over anhydrous sodium sulfate. Recover diethyl ether, distill under reduced pressure, and collect fractions at 94-97°C/17kPa to obtain 215g of 2,2,2-trichloroethanol (1), yield 84%, solidified after cooling, mp 16-18°C. [4]
Purpose
1. Used in organic synthesis and pharmaceutical intermediates.
2.Prepare trichloroethanol ester to protect the carbonyl group and protect the carboxyl group in peptide synthesis.

 微信扫一扫打赏
微信扫一扫打赏

