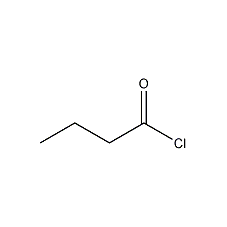
Structural formula
| Business number | 03U8 |
|---|---|
| Molecular formula | C4H7ClO |
| Molecular weight | 106 |
| label |
n-Butyryl chloride, butyryl chloride, n-butyryl chloride, Butanoyl chloride, n-Butyl chloride, Pesticide intermediates; aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:141-75-3
MDL number:MFCD00000752
EINECS number:205-498-5
RTECS number:None
BRN number:605395
PubChem ID:None
Physical property data
1. Properties: colorless, clear and transparent liquid. It has the pungent smell of acid chloride.
2. Density (g/mL, 25/4℃): 1.0263
3. Relative density (20℃, 4℃): 1.0277
4 . Melting point (ºC): -89
5. Boiling point (ºC, normal pressure): 102
6. Refractive index at room temperature (n20): 1.4121
7. Refractive index: 1.412
8. Flash point (ºC): 21
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Miscible with ether. Slowly dissolves in water and ethanol and gradually decomposes.
Toxicological data
None yet
Ecological data
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 25.45
2. Molar volume (cm3/mol): 103.0
3. Isotonic specific volume (90.2K ): 234.7
4. Surface tension (dyne/cm) 26.9
5. Polarizability (10-24cm3): 10.09
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. RotatableNumber of scientific bonds: 2
5. Number of tautomers: None
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 51.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
Incompatible materials: water, strong alkalis, alcohols, strong oxidants.
2.This product is toxic. It is highly toxic when swallowed and absorbed and is highly irritating to tissues. Production equipment should be sealed to prevent leakage. The site must have good ventilation conditions. Operators must wear protective equipment.
Storage method
Store sealed in a dry and cool place. Packed in glass bottles and required to be airtight. Store in a cool and ventilated place, away from rain, moisture, sun and impact. Store and transport according to regulations on toxic substances.
Synthesis method
1. Obtained from the reaction of butyric acid and thionyl chloride. Heat thionyl chloride to reflux, add butyric acid dropwise, finish adding in about 4 hours, reflux for another 1 hour, distill, collect the 95-115°C fraction, re-distill once, collect the 101-105°C fraction to get the finished product, collect The rate is over 70%. Phosphorus trichloride can also be added in the reaction, and the yield is 75%. The purity of industrial products is ≥99%. Raw material consumption quota: butyric acid 890kg/t, thionyl chloride 600kg/t.
![]()
2. Its preparation method is: In the reactor, heat thionyl chloride to reflux, add n-butyric acid dropwise, complete the addition in about 4 hours, then reflux for another 1 hour, collect the 95-115°C fraction into crude butyryl chloride by distillation under normal pressure, and further distill it to obtain the product.
Purpose
1. Used in organic synthesis.
2. Butyryl chloride is an intermediate of the herbicides sethlopidine, trimetrione, and dimethiodone.
3. Organic synthesis intermediates, used for the preparation of butylbenzene and the drugs diuric acid, diuretic acid and intermediate 2-ethyl-1,3-cyclopentanedione.

 微信扫一扫打赏
微信扫一扫打赏

