
Structural formula
| Business number | 03VQ |
|---|---|
| Molecular formula | C12H24O2 |
| Molecular weight | 200.32 |
| label |
n-Dodecanoic acid, dodecanoic acid, n-Dodecanoic acid, Softeners, plasticizers, stabilizers, acidic solvent |
Numbering system
CAS number:143-07-7
MDL number:MFCD00002736
EINECS number:205-582-1
RTECS number:OE9800000
BRN number:1099477
PubChem number:24896407
Physical property data
1. Properties: White needle-shaped crystals with a slight laurel oil scent.
2. Density (g/mL, 50℃): 0.8679
3. Saturated vapor pressure (kPa, 121ºC): 0.133
4. Melting point ( ºC): 44~46
5. Boiling point (ºC, normal pressure): 225 (13.3kpa)
6. Flash point (ºC): >110
7. Refractive index (n82D): 1.4183 (82℃), 1.4323 (45℃)
8. Solubility: Insoluble in water, soluble in methanol, ether, chloroform and other organic solvents, slightly soluble in acetone and petroleum ether.
9. Refractive index at room temperature (n25): 1.430450
10. Relative density (25℃, 4℃ ): 0.867550
11. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -7510.11
12. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -641.95
13. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -7414.0
14. The liquid phase standard claims heat (enthalpy) (kJ·mol-1): -738.1
15. The standard heat of combustion (enthalpy) of the crystal phase (kJ·mol-1): -7377.48
16. The standard claim heat (enthalpy) of the crystal phase (kJ· mol-1):-774.58
Toxicological data
1. Acute toxicity: rat oral LD50: 12 gm/kg; mouse intravenous LC50: 131 mg/kg.
2. Chronic toxicity/carcinogenicity: Transdermal TCLo in mice: 108 gm/kg/15W-I It is irritating to the eyes, skin, mucous membranes and upper respiratory tract. Taking large amounts orally can cause gastrointestinal discomfort.
Ecological data
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 59.20
2. Molar volume (cm3/mol): 221.2
3. Isotonic specific volume (90.2K ): 531.3
4. Surface tension (dyne/cm): 33.2
5. Polarizability (10-24cm3): 23.47
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 4.2
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 10
5. Topology Molecular polar surface area (TPSA): 37.3
6, Number of heavy atoms: 14
7, Surface charge: 0
8, Complexity: 132
9. Number of isotope atoms: 0
10. Number of determined atomic stereocenters: 0
11. Number of uncertain atomic stereocenters: 0
12. Determined number of stereocenters of chemical bonds: 0
13. Uncertain number of stereocenters of chemical bonds: 0
14. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure. Incompatible materials: alkali, oxidizing agent, reducing agent.
Colorless needle-shaped crystals. Soluble in methanol, slightly soluble in acetone and petroleum ether. Insoluble in water. It has a sulfation effect with concentrated sulfuric acid and has no chemical effect when exposed to strong alkali.
2.This product is non-toxic. Little irritation to skin.
3. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves, and smoke.
4. Naturally found in laurel oil and coconut oil.
Storage method
1. Packed in a plastic bag wrapped in a gunny bag. Store in a cool, ventilated and dry place. Keep away from heat sources and fires, and protect against moisture and sun.
2. Packed in plastic bags and gunny bags, 25kg or 50kg per bag. Store and transport according to general chemical regulations.
Synthesis method
1. Industrial production methods can be summarized into two categories: one is obtained from natural vegetable oils through saponification or decomposition under high temperature and pressure; the other is separation from synthetic fatty acids. Japan mainly uses coconut oil and palm kernel oil as raw materials to produce lauric acid. Natural vegetable oils used to prepare lauric acid include: coconut oil, litsea cubeba kernel oil, palm kernel oil and mountain pepper kernel oil. Other plants, such as palm kernel oil, tree seed oil, camphor tree seed oil, etc., can also be used to produce lauric acid in the service industry. The C12 fraction remaining after extracting dodecanoic acid contains a large amount of dodecenoic acid, which can be hydrogenated at normal pressure without a catalyst and can convert hydrocarbons into dodecanoic acid with a conversion rate of more than 86%.
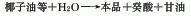
2. From coconut oil and others Vegetable oil is separated and refined after hydrolysis.

3. Natural in the form of glycerides Exist in coconut oil, litsea cubeba kernel oil, palm kernel oil and pepper kernel oil. Industrially, it can be obtained from the hydrolysis of natural oils. Add coconut oil, water and catalyst to the autoclave, and hydrolyze it into glycerin and fatty acids at 250°C and about 5MPa pressure, which contain 45% to 80% dodecanoic acid. Dodecanoic acid is obtained by distillation.
4.Palm kernel oil method Malaysia’s palm oil production accounts for more than half of the world’s total production. Among them, palm kernel oil is mainly composed of C6 to C18 fatty acids, usually lauric acid accounts for 46% to 51%.
5.Peppercorn Kernel Oil Method Pipermum nidus belongs to the Lauraceae family and is a deciduous tree. The drupe of mountain pepper is spherical. The fruit is green at first and turns black when mature. The kernel oil of mountain pepper contains more than 30% lauric acid.
Other plants, such as palm kernel oil, sassafras seed oil, camphor tree seed oil, etc. can also be used to prepare lauric acid.
6. Tobacco: OR, 44; FC, 41; OR, 26; FC, OR, 18; FC, 15; BU, 26; FC, 40.
Purpose
1. Mainly used as raw materials for the production of alkyd resins, wetting agents, detergents, pesticides, surfactants, food additives and cosmetics. This product is often used as a lubricant and has multiple functions such as lubricant and vulcanizing agent. However, due to its corrosive effect on metal, it is generally not used in plastic products such as wires and cables. This product is most widely used in surfactant industry, and can also be used in spice industry and pharmaceutical industry.
2.Used as surface treatment agent for preparing bonding. It is also used in the manufacture of alkyd resins, chemical fiber oils, pesticides, synthetic fragrances, plastic stabilizers, anti-corrosion additives for gasoline and lubricating oils. A large number of surfactants are used in the manufacture of various types of surfactants, such as cationic types include laurylamine, lauryl nitrile, trilaurylamine, lauryl dimethylamine, lauryl trimethyl ammonium salt, etc.; anionic types include sodium lauryl sulfate, Lauric acid sulfate salt, lauryl sulfate triethanolammonium salt, etc.; zwitterionic types include lauryl betaine, lauric acid imidazoline, etc.; non-ionic surfactants include poly-L-alcohol monolaurate, polyoxyethylene laurate, etc. Acid ester, lauric acid glyceryl polyoxyethylene ether, lauric acid diethanolamide, etc. In addition, it is also used as a food additive and in the manufacture of cosmetics.
3.Lauric acid is a raw material for the production of soaps, detergents, cosmetic surfactants and chemical fiber oils.

 微信扫一扫打赏
微信扫一扫打赏

