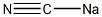
Structural formula
| Business number | 03W3 |
|---|---|
| Molecular formula | NaCN |
| Molecular weight | 49.01 |
| label |
kaempferol, : Shannai, Cianuro disodio, mordant, whitening agent, masking agent, quenching agent, Raw materials for printed circuit board electroplating |
Numbering system
CAS number:143-33-9
MDL number:MFCD00003523
EINECS number:205-599-4
RTECS number:VZ7525000
BRN number:3587243
PubChem number:24886191
Physical property data
1. Characteristics: White or slightly colored lumpy or crystalline particles with a faint bitter almond smell. [9]
2. Melting point (℃): 563.7[10]
3. Boiling point (℃): 1496 [11]
4. Relative density (water = 1): 1.596[12]
5. Saturated vapor pressure (kPa): 0.13 (817℃)[13]
6. Octanol/water partition coefficient: -1.69[14]
7. Solubility: Easily soluble in water, soluble in liquid ammonia, slightly soluble in ethanol, ether and benzene. [15]
Toxicological data
1. Acute toxicity:
Rat oral LD50: 6440 ug/kg; rat abdominal LD50: 4300 ug/kg; mouse abdominal LD50: 4900 ug/kg; mouse subcutaneous LD50 : 3600 ug/kg; rabbit transdermal LD50: 10400 ug/kg; rabbit subcutaneous LD50: 2200 ug/kg.
2. Acute toxicity [16] LD50: 6.4mg/kg (rat oral)
3. Irritation No data yet
4. Others[17] The lowest toxic dose for hamster implantation (TDLo): 5999mg/ kg (6~9 days of pregnancy), causing embryotoxicity, abnormal musculoskeletal development and abnormal development of the cardiovascular (circulatory) system.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[18] This substance is harmful to the environment and should be treated with special Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: not available
2. Molar volume (cm3/mol): not available
3. etc. Zhang specific volume (90.2K): None available
4. Surface tension (dyne/cm): None available
5. Dielectric constant: None available
6. Polarizability (10-24cm3): None available
7. Single isotope mass: 48.992844 Da
8. Nominal mass: 49 Da
9. Average mass: 49.0072 Da
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2.�ternal/day_100531/201005311400497712.gif” alt=”” />
The tail gas after water absorbs hydrogen cyanide contains 65% hydrogen and about 30% carbon monoxide, which can be recycled to produce ammonia, methanol, oxalic acid and other products.
(5) Light oil cracking method: Mix light oil and ammonia in an atomizer in proportion, preheat to 280°C, conduct a cracking reaction in an electric arc furnace, use petroleum coke as a carrier and nitrogen as a protective gas for airtight anti-oxidation. At a temperature of 1450°C, the reaction generates hydrocyanic acid gas. After dust removal and cooling, pure hydrocyanic acid is obtained by removing ammonia, washing with water, absorbing, and distilling, and then absorbing it with alkali liquid to generate sodium cyanide. The reaction is as follows: ![]()
(6) Acrylonitrile by-product process in propylene ammoxidation process In the process of acrylonitrile production, hydrogen cyanide gas is produced as a by-product (the amount is equivalent to 4% to 10% of acrylonitrile production), which is absorbed by alkali liquid, and then evaporated, concentrated, separated, and dried to obtain sodium cyanide product. Its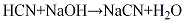
Put 1L of NaOH containing no more than 40g. Cool the water-ethanol solution with ice. Add a slight excess of liquid hydrogen cyanide to the solution. If the solution is left for a long time, crystals will precipitate, filter it, and dry it under reduced pressure in a P2O5 dryer.
Purpose
1. Used as a quenching agent for various steels, the main component of copper plating, silver, cadmium, zinc, etc., used to extract precious metals such as gold and silver, and also a raw material for cyanide and hydrocyanic acid.
2. Used as a quenching agent for various steels in the machinery industry. In the electroplating industry, it is used as the main component of copper, silver, cadmium and zinc plating. In the metallurgical industry, it is used to extract precious metals such as gold and silver. In the chemical industry, it is a raw material for manufacturing various inorganic cyanides and hydrocyanic acid. It is also used to manufacture copolymers of organic glass, various synthetic materials, nitrile rubber, and synthetic fibers. In the dye industry, it is used to make cyanuric chloride (reactive dye intermediate and raw material for the production of brighteners). In the pharmaceutical industry, it is used to manufacture methyl cyanoacetate and diethyl malonate. It is used as a mordant in the textile industry and is also used in liquid carburizing and nitriding of steel.
The important inorganic cyanides produced directly from sodium cyanide mainly include sodium cyanide, potassium cyanide, zinc cyanide, barium cyanide, cuprous cyanide, and thiocyanide. Sodium, potassium thiocyanide; organic cyanides include cyanoacetic acid, malononitrile, methionine, benzyl cyanide, melamine chloride, etc. The main products that use sodium cyanide to reproduce hydrogen cyanide are: methyl methacrylate, butyl methacrylate, methacrylic acid, azobisisobutyronitrile, azobisisoheptanitrile, nitrilotriacetic acid, hydroxyl Acetonitrile etc.
3. It is used as a masking agent in analysis, as a chromium mixture in the smelting and electroplating industries, and in the research of insect hormones.
4. Used in plastics, medicine, pesticides, dyes, metallurgy, electroplating, mineral processing and other industries, and also used as raw material for producing hydrocyanic acid. Complexing agent. Masking agent. Used for refining gold and silver ore.
5. Sodium cyanide in the electroplating solution can reduce the anode polarization, ensure the normal dissolution of the anode, stabilize the plating solution and improve the cathode polarization to obtain a uniform coating.
6. Sodium cyanide can be used to prepare cyano compounds [2,3], cyanohydrins [4,5], α-Aminocyano compound [6] can also be used as a catalyst, such as benzoin condensation reaction.
Synthesis of cyano compounds In a suitable solvent, cyanide can be obtained by reacting active alcohol or halide with sodium cyanide. This reaction is a nucleophilic substitution reaction and is cyanide. General methods for chemical synthesis. In DMSO, when sodium cyanide reacts with alkyl chloride, sodium cyanide has a higher reaction yield than potassium cyanide, and the reaction time is shorter (Formula 1)[2].
![]()
Under microwave conditions, cyanidation The reaction of sodium with aryl halides is a rapid method for preparing aryl cyanides (Formula 2)[3]. The reaction is fast and the yield is high, making it a good method for synthesizing aryl cyanides.

Cyanohydrins Synthesis In the process of synthesizing cis- and trans-hydroxyglimepiride, Gurjar and Joshi et al. used β-ethyl carbonyl butyrate as raw material to generate nitrile through a two-step reaction. Alcohol compounds have a higher yield (formula 3)[4].

α Synthesis of -cyanoamino compound In the presence of ruthenium catalysis and oxygen, the reaction of tertiary amine and sodium cyanide produces α-cyanoamino compound (Formula 4)[7], the solvent is a mixture of methanol and acetic acid (3:1). The tertiary amine obtained by oxidative cyanation is the precursor for the synthesis of α-amino acids. Therefore, this method provides a quick and convenient way to synthesize α-amino acids.

Ring formation Sodium cyanide can be used as a catalyst for the dimerization of imine compounds into quinoxaline or pyrazine. This reaction is similar to benzoin condensation (Formula 5)[8]. In DMF, methanol or methylene chloride/water (phase transfer conditions), sodium cyanide rapidly promotes intramolecular cyclization to give six-membered rings.

7. Used to refine gold, Silver and other precious metals are quenched and used in organic synthesis industries such as plastics, pesticides, medicines, and dyes. [24]
src=”http://images.basechem.org/internal/day_120508/201205081517426620.jpg” alt=”” />
7. Used for refining and quenching gold, silver and other precious metals, and for Plastics, pesticides, medicines, dyes and other organic synthesis industries. [24]

 微信扫一扫打赏
微信扫一扫打赏

