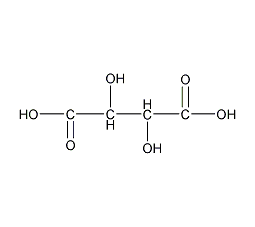
Structural formula
| Business number | 03X6 |
|---|---|
| Molecular formula | C4H6O6 |
| Molecular weight | 150.09 |
| label |
L-glutonic acid; dihydroxysuccinic acid, D-tartaric acid, sour grapes, D-tartaric acid, Unnatural tartaric acid, D-Tartaric acid, D-threo-2,3-Dihy-droxysuccinic acid, [S-(R*,R*)]-2,3-Dihydroxybutanedioic acid, acidic solvent |
Numbering system
CAS number:147-71-7
MDL number:MFCD00004238
EINECS number:205-695-6
RTECS number:WW7875000
BRN number:1725145
PubChem number:24900012
Physical property data
1. Properties: Monoclinic prismatic crystal.
2. Density (g/mL, 20℃): 1.7598 3. Melting point (ºC): 172.5 4. Flash point (ºC): 210 5. Specific rotation (º): -12.1 6. Solubility: Soluble in water, boiling water, methanol, ethanol, propanol, ether, soluble in glycerin and insoluble in chloroform. 7. Refractive index (n20D): 1.4955
Toxicological data
The median lethal dose (mouse, intravenous) is 485 mg/kg. irritating
Ecological data
3. Ecological data:
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 26.69
2. Molar volume (cm3/mol): 79.5
3. Isotonic specific volume (90.2K): 263.1
4. Surface tension (dyne/cm): 119.4
5. Polarizability (10-24 cm3): 10.58
Compute chemical data
IV. Calculated chemical data:
1. Hydrophobic parameter calculation reference value (XlogP): -1.9
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 3
5. Topological molecular polar surface area (TPSA): 115
6. Number of heavy atoms: 10
7. Surface charge: 0
8. Complexity: 134
9. Number of isotope atoms: 0
10. The number of determined atomic stereocenters: 2
11. The number of uncertain atomic stereocenters: 0
12�� Determined number of stereocenters of chemical bonds: 0
13, Uncertain number of stereocenters of chemical bonds: 0
14, Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
2. Incompatible materials: strong oxidizing agents.
3. Found in tobacco leaves.
Storage method
Store sealed in a dry and cool place.
Synthesis method
Tartaric acid mainly exists in the fruits of various plants in the form of potassium salt, and a small amount also exists in the free state. D-tartaric acid is commonly produced in industry by fermenting glucose; the racemate can be produced by oxidizing fumaric acid with potassium permanganate; the mesoform can be produced by oxidizing maleic acid with potassium permanganate; L-alcolic acid can be obtained from the racemate. In the field of practical application of tartaric acid, dextrorotatory tartaric acid or its double salt is mainly used. Tartar, a by-product of grape brewing, is the main raw material for the actual production of tartaric acid at present, and the tartaric acid produced is all dextrorotatory.
Purpose
1. Tartaric acid is widely used as an acidifier in beverages and other foods. This use is similar to that of citric acid. Tartaric acid, combined with tannins, can be used as a mordant for acid dyes and is also used in certain developing and fixing operations in the photographic industry. Its iron salts are photosensitive and therefore can be used to make blueprints. Tartaric acid can complex with a variety of metal ions and can be used as a cleaning agent and polishing agent for metal surfaces. Potassium sodium tartrate (Rochelle’s salt) can be used to prepare Fehling’s reagent, and can also be used in medicine as a laxative and diuretic, and as an intermediate for cinchofen. Its crystals have piezoelectric properties and can be used in the electronics industry.
2. Used as chromatographic analysis reagents and masking agents.
3. Used as a pharmaceutical resolving agent, food additive, biochemical reagent, etc. Usage: This product is widely used in the food industry, such as as a beer foaming agent, food sour agent, flavoring agent, used in refreshing beverages, Candy, juice, sauce, cold dishes, baking powder, etc. This product complies with the Japanese Food Additives Convention. Biochemical research.

 微信扫一扫打赏
微信扫一扫打赏

