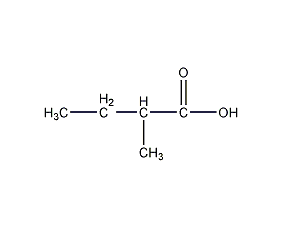
Structural formula
| Business number | 038Q |
|---|---|
| Molecular formula | C5H10O2 |
| Molecular weight | 102.13 |
| label |
o-Methylbutyric acid, a-methylbutyric acid, 2-Methylbutanoic acid, Active valeric acid, a-Methylbutyric acid, food additives, flavor enhancer, acidic solvent |
Numbering system
CAS number:116-53-0
MDL number:MFCD00002669
EINECS number:204-145-2
RTECS number:EK7897000
BRN number:1720486
PubChem number:24884519
Physical property data
1. Properties: Colorless to light yellow liquid, with a pungent and spicy Roquefort cheese smell. At low concentrations, it has a pleasant fruity aroma and a spicy and spicy taste.
2. Density (g/mL, 25℃): 0.938
3. Relative density (20℃, 4℃): 0.9410
4. Melting point (ºC): -70
5. Boiling point (ºC, normal pressure): 177
6. Boiling point (ºC, 2.8KPa): 84
7 . N room temperature refractive index (n 20 ): 1.4051
8. Flash point (ºc): 73
9. kJ·mol-1): -2902.9
10. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -493.7
11. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2842.2
12. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -554.4
13. Liquid phase standard hot melt (J·mol-1·K– 1): 217.0
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16 . The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. The lower limit of explosion (%) ,V/V): Undetermined
19. Solubility: Slightly soluble in water and glycerin, soluble in ethanol and propylene glycol.
Toxicological data
1. Acute toxicity: Rat oral LD5O: 1870mg/kg
Rabbit transdermal LD5O: 1460ul/kg
Ecological data
Slightly harmful to water.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 26.73
2. Molar volume (cm3/mol): 106.0
3. Isotonic specific volume (90.2K): 250.2
4. Surface tension (dyne/cm): 30.9
5. Polarizability (10-24cm3): 10.59
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.2
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 37.3
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 68.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters Quantity: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable at room temperature and pressure, avoid contact with strong oxidants, alkalis, and reducing agents.
2. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
3. Naturally found in apricots, beer, pear brandy and wine.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire, heat sources and anti-static. The packaging is sealed. It should be stored separately from strong alkalis, acids, and reducing agents, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Obtained from the oxidation of fusel oil. It is derived from the heated decarboxylation of methylethylmalonic acid.
2. Tobacco: OR, 44; OR, 49; Bu, 56; OR, 26; FC, 9; FC, BU, OR, 18; Bu, 9; OR, 41; BU, 26 ;FC,40/.
3. In a reaction bottle equipped with a stirrer, thermometer, reflux condenser, and dropping funnel, add 12.5g of clean magnesium chips, 50mL of anhydrous ether, one grain of iodine, and 3g of 2-chloroprene. alkane and heat slowly. After the reaction started, 75 mL of anhydrous ether was added. Add dropwise a solution composed of 43g of 2-chlorobutane (2) (0.5mol in total) and 270mL of anhydrous ether. Control the reflux slowly and finish adding in about 0.5h. Then continue to stir and reflux until the magnesium reaction is basically completed. Add 100 mL of anhydrous ether, and cool the reaction to about -12°C in an ice-salt bath. Slowly pour the reactants into 125g of dry powdered dry ice and stir thoroughly until the dry ice evaporates completely. Then slowly add a mixture of 300g crushed ice and 75mL concentrated hydrochloric acid, and stir thoroughly to separate completely. The organic layer was separated, the aqueous layer was extracted with diethyl ether, and the organic layers were combined. Add crushed ice to the ether layer, add 100 mL of 20% sodium hydroxide solution, and shake thoroughly to allow the organic acid to enter the water layer. The aqueous layer was separated, and the ether layer was extracted with sodium hydroxide solution. Combine the water layers. The aqueous layer was concentrated under reduced pressure. Cool and acidify with hydrochloric acid. The organic layer was separated, and the aqueous layer was distilled until no oil was distilled out. Saturate the eluate with sodium chloride, separate the organic layers, combine the organic layers, dry with anhydrous sodium sulfate, and distill. Collect the fractions at 173-174°C to obtain 40g of 2-methylbutyric acid (1), with a yield of 79%. . [1]
Purpose
1. Used for preparing edible, tobacco and daily chemical flavors.
2. Used in baked goods, beverages, and candies.

 微信扫一扫打赏
微信扫一扫打赏

