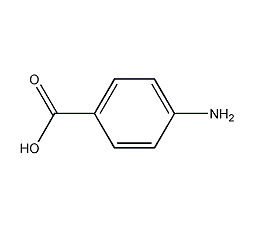
Structural formula
| Business number | 03Y1 |
|---|---|
| Molecular formula | C7H7NO2 |
| Molecular weight | 137.14 |
| label |
4-aminobenzoic acid, Vitamin Bx, 4-Aminobenzoic acid, PABA, Amben, Anticanitic vitamins, Antichromotrichia factor, Bacterial vitamin H1, Chromotrichia factor, Vitamin Bx, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:150-13-0
MDL number:MFCD00007894
EINECS number:205-753-0
RTECS number:DG1400000
BRN number:471605
PubChem ID:None
Physical property data
1. Properties: colorless needle-shaped crystals, turning into light yellow in the air or under light. The industrial product is beige or off-white paste.
2. Melting point (℃): 187-187.5
3. Relative density (g/mL, 25/4℃): 1.374
4. Dissolution Properties: Easily soluble in hot water, ether, ethyl acetate, ethanol and glacial acetic acid, insoluble in water, benzene, and insoluble in petroleum ether.
Toxicological data
1. Acute toxicity: Rat oral LD50: >6 gm/kg;
Rat abdominal LD50: >3450 mg/kg;
Mouse oral LC50 : 2850 mg/kg;
Rabbit oral LD50: 1830 mg/kg.
Ecological data
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 37.41
2. Molar volume (cm3/mol): 104.2
3. Isotonic specific volume (90.2K): 295.2
4. Surface tension (dyne/cm): 64.3
5. Polarizability (10-24cm3): 14.83
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecule polar surface area 63.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 128
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
Incompatible materials: Strong oxidants
2. Moderately toxic.Irritating skin and mucous membranes. After contact with skin, rinse immediately with water. The production workshop should be well ventilated and the equipment should be sealed. Operators should wear protective equipment.
Storage method
1. During storage, it should be protected from heat, moisture and sunlight, and placed in a cool, ventilated and dry place.
2. Packed in polyethylene-lined plastic bags and outer sacks, 50kg per bag. Store in a dry, ventilated place. Store and transport according to regulations for flammable and toxic chemicals.
Synthesis method
1. Add water, sodium chloride, and hydrochloric acid to the reaction pot in sequence. Start stirring and raise the temperature to 50-60°C, add 9/10 of the iron powder, continue to raise the temperature to 98-102°C, keep warm for 1 hour, stop heating, slowly add p-nitrobenzoic acid at 95°C, about 1-1.5 hours After adding, add iron powder, keep it at 98-102℃ for 1 hour, cool to 80℃, add 30% liquid caustic soda to adjust pH=8.5-9, press filter, wash with a small amount of water, combine the filtrate with a small amount of washing liquid, and press Acid precipitation pot, cool to 30-34°C, add insurance powder to decolorize, and filter. Add acid to the filtrate to adjust pH to 3.5-4, continue to cool to below 10°C, remove water and dry to obtain the finished product. The yield is 84-88%. (2) Obtained by catalytic hydrogenation of p-nitrobenzoic acid. Add industrial p-nitrobenzoic acid and water to a 1000ml beaker, adjust pH=6-7 with 22% sodium hydroxide under stirring, and then add Ruan Ni Nickel, pump the material liquid into the swing autoclave under stirring, replace it twice with hydrogen and nitrogen respectively, then pressurize to 3.43MPa, react at a pressure of 3.43-2.54MPa and a temperature of 134-140°C for about 5 hours. The end point is when the hydrogen pressure does not drop. After the reaction is completed, filter and dry to obtain the finished product. The yield is over 80%.
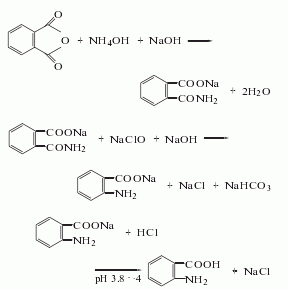
2.There are two main methods of industrial production.
(1) Para-ester benzoic acid is reduced by iron powder. The reaction formula is as follows:
![]()
(2) It is obtained by catalytic hydrogenation of p-nitrobenzoic acid. The reaction formula is as follows:
![]()
Purpose
1. Used as pharmaceutical and dye intermediates.
2. Dyes and pharmaceutical intermediates. It is used to produce reactive red M-80, M-10B, reactive red violet X-2R and other dyes and to prepare cyanobenzoic acid to produce the drug p-carboxybenzylamine. Para-aminobenzoic acid can be used as a sunscreen agent, and its derivative, octyl p-dimethylcarbamate, is an excellent sunscreen agent with the trade name Padimate O.
3. Used as analytical reagents, sunscreen agents, and also in the pharmaceutical and dye industries.
4. In addition to p-aminobenzoic acid, this product also contains the physical sunscreen agent titanium dioxide, so it has the characteristics of low irritation and high safety. .
5. Calibration of alkali normality, reagents for determination of copper, synthesis of esters, folic acid and azo dyes.

 微信扫一扫打赏
微信扫一扫打赏

