Overview[1]
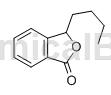
Butyl phthalide is divided into n-butyl phthalide and butylene phthalide. The chemical name of n-butyl phthalide is 3-butyl-1(3H)-isobenzofuranone, also known as apigenin. , the trade name is Enbipu, it belongs to the simple phthalides, is an oily liquid, and has the aroma of celery. It is a drug independently researched and developed in my country for the treatment of ischemic stroke. It has an excellent safety single structure. , which also has a variety of pharmacological effects, can comprehensively treat ischemic stroke, significantly reduce post-infarction neurological deficits, and improve patients’ living ability status. Because of its above advantages, it has attracted widespread attention.
Butylidenephthalide (BP) can effectively inhibit the expression of growth factors, stemness factors, epithelial-mesenchymal transition (EMT) factors, and gelatinase of cancer stem cells. , especially can effectively inhibit the expression of Sox-2, Oct4, and EZH2, so it can inhibit the growth, migration, invasion, and metastasis of cancer stem cells, and thus can be used to prevent, slow down, and/or inhibit the growth, migration, and invasion of cancer stem cells. and/or transferred pharmaceuticals, foods, or food additives.
Apply[3]
Butylphthalide, commonly known as apigenin A, is the main component of celery volatile oil. It is also widely found in other plants of the Apiaceae and Asteraceae families. Apigenin A has strong anti-convulsant, anti-asthmatic, and inhibitory effects. Prostaglandin F2x has various pharmacological activities such as proliferation, increased blood flow, anti-tumor and antihypertensive activities, indicating that it has broad clinical application prospects. In addition, apigenin A has a natural banana aroma and can also be used in the food industry.
The applications of butylidenephthalide (BP) include preventing, slowing down and/or inhibiting cancer stem cells (CSCs), especially oral cancer stem cells, nasopharyngeal cancer stem cells, esophageal cancer stem cells, myeloma stem cells, Skin cancer stem cells, melanoma stem cells, thyroid cancer stem cells, lymphoma stem cells, blood cancer stem cells, breast cancer stem cells, bladder cancer stem cells, ovarian cancer stem cells, cervical cancer stem cells, especially oral cancer stem cells, pancreatic cancer stem cells, and brain tumor stem cells ( The growth, migration, invasion and/or metastasis of braintumor stem cells.
Preparation[2]
Method 1:
1. Preparation of Grignard reagent
Add 11.04g (0.46mol) magnesium strip into a 500ml four-neck bottle, add one grain of elemental iodine and 50ml ether, stir, add about 2g n-butyl bromide, and slowly add the remaining n-butyl bromide dropwise after the reaction is initiated. Add a total of 63.02g (0.46mol) of bromine and n-butyl bromide, and add 200ml of isopropyl ether at the same time. Complete the dropwise addition in about 1 hour, then reflux for 1 hour, cool down, and set aside.
2. Crude product preparation
Add 58.40g (0.4mol) o-cyanobenzaldehyde into a 1000ml four-neck bottle, add 300ml isopropyl ether, stir, add 1.0g cuprous iodide, cool to -25°C, and control the temperature to -25± Add the above-mentioned Grignard reagent dropwise at 2°C, complete the dropwise addition in about 1 hour, keep stirring at -20±2°C for 2 hours, add 10% hydrochloric acid dropwise, adjust the pH to 2~3, complete the dripping, heat to reflux for 2 hours, and separate the water. layer, the organic layer was washed twice with 200ml of 1% hydrochloric acid, the organic layer was added with sodium hydroxide solution (32g sodium hydroxide added to 500ml of water), heated to reflux for 1 hour, cooled, separated, and the aqueous layer was washed twice with 200ml of isopropyl ether. , the water layer is returned to the reaction bottle, adjust the pH to 3~4 with concentrated hydrochloric acid, react at 70~80°C for 1 hour, cool down, add isopropyl ether to extract twice (100ml*2), and then wash the isopropyl ether phase with 100ml water Concentrate twice to obtain 63.54g of crude product, with a yield of 83.6%.
3. Distillation
Add 63.5g of the crude product into a 100ml reaction bottle, add 0.4g of sodium bicarbonate, heat in an oil bath at 160-180°C, reduce the pressure, and reach a vacuum below 100pa to obtain 49.8g of n-butylphthalide with a single heterogeneity of less than 0.1%. , yield 78.4%, purity 99.79%.
Method 2:
Put 60.0g of phthalic anhydride, 113.2g of valeric anhydride and 33.23g of anhydrous sodium acetate into a 500ml reaction bottle, raise the temperature to 200°C and react for 3 hours. After the reaction is completed, concentrate under reduced pressure to recover the generated n-valeric acid. Cool to 30°C and slowly add boron. Sodium hydroxide and sodium hydroxide solution (6.5g sodium borohydride, 40.0g sodium hydroxide dissolved in 100ml water), react at 60°C for 5 hours. After the reaction is completed, dichloromethane extracts impurities. Use dilute hydrochloric acid to adjust the pH of the water phase to 1, 85°C. Incubate for 3 hours, cool down, extract with dichloromethane, and concentrate to obtain 54.2g of n-butylphthalide, with a yield of 70.33% and a liquid phase purity of 98.36%.
Main reference materials
[1] Feng Yipu, Hu Dun, & Zhang Liying. (1995). Protective effect of butylphthalide on global cerebral ischemia in mice. Acta Pharmaceutical Sinica (10), 741-744.
[2] Xiong Jie, & Feng Yipu. (1999). Effects of butylphthalide on the activity of mitochondrial respiratory chain complex enzymes. Acta Pharmaceutical Sinica, 34(4), 241-245.
[3] Xiong Jie, Xu Pingxiang, Sun Lina, Cheng Liang, Feng Yipu, & Wang Xiaoliang. (2007). Protective effect of butylphthalide on mitochondrial function of primary cultured neurons. Traditional Chinese Medicine Pharmacology and Clinical Medicine, 23(5) , 73-76.

 微信扫一扫打赏
微信扫一扫打赏

