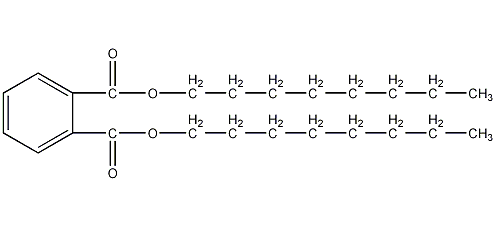
Structural formula
| Business number | 039C |
|---|---|
| Molecular formula | C24H38O4 |
| Molecular weight | 390.56 |
| label |
Dioctyl phthalate, n-Octyl phthalate, Dioctyl o-benzenedicarboxyolate, plasticizer, condensing agent, Gas Chromatography Stationary Solution |
Numbering system
CAS number:117-84-0
MDL number:MFCD00015292
EINECS number:204-214-7
RTECS number:TI1925000
BRN number:1915994
PubChem number:24862966
Physical property data
1. Properties: colorless, odorless, high viscosity liquid.
2. Density (g/mL, 20/4℃): 0.9786
3. Melting point (ºC): 25
4. Boiling point (ºC, Normal pressure): 220 (533.2pa)
5. Boiling point (ºC, 0.67KPa): 231
6. Refractive index (25ºC): 1.483
7. Flash point (ºC): 219
8. Vapor pressure (kPa, 150ºC): <0.027
9. Viscosity (mPa·s, 30ºC): 24.5
10. Solubility (%, water): <0.02
11. Solubility: poorly soluble in water, cellulose acetoacetate, cellulose nitrate, polymethylmethacrylate, polymethylmethacrylate Styrene, vinyl chloride-vinyl acetate copolymer, polyvinyl chloride, paraffin oil, dammar resin, coumarone resin, etc. have strong dissolving ability. Polyvinyl acetate and shellac are difficult to dissolve, but cellulose acetate is insoluble.
12. Refractive index at room temperature (n20): 1.4855
13. Relative density (25℃, 4℃): 0.974724
Toxicological data
1. Irritation: Rabbit transdermal: 500mg/24 hours, mild irritation. Rabbit eye: 500mg/24 hours, mild irritation.
2. Acute toxicity: Mouse oral LD50: 6513mg/kg; Mouse abdominal LD50: 65g/kg;
Mouse inhalation LC50: 5000μg/m3; Guinea pig transdermal LD50: >5mL/kg.
3. Mild irritation to eyes.
Ecological data
This substance is harmful to the environment and it is recommended not to let it enter the environment.
Molecular structure data
1. Molar refractive index: 114.65
2. Molar volume (cm3/mol): 396.3
3. Isotonic specific volume (90.2K ): 973.9
4. Surface tension (dyne/cm): 36.4
5. Polarizability (10-24cm3): 45.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 18
5. Number of tautomers: none
6. Topological molecule polar surface area 52.6
7. Number of heavy atoms: 28
8. Surface charge: 0
9.���Hurility: 369
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain atomic stereocenter Number of stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Total Number of price key units: 1
Properties and stability
Has a special smell. The ignition point is 241℃, the viscosity is 81.4mPa·s, and the vapor pressure (200℃) is 176Pa. The solubility of this product in water is < 0.01% at 25°C, and the solubility of water in this product is 0.2%. Soluble in most organic solvents and hydrocarbons, slightly soluble in glycerin and ethylene glycol. Has good compatibility with most industrial resins. Partially compatible with cellulose acetate; polyvinyl acetate.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and water sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Produced by esterification of phthalic anhydride and n-octanol under normal pressure. Add phthalic anhydride, n-octanol, benzene, and sulfuric acid to the reaction kettle, and slowly heat up while stirring until benzene and water begin to steam out. After the benzene-water mixture is condensed by the condenser, the water is separated by the benzene-water separator, and the benzene is returned to the kettle to continue evaporation and water. When no water is brought out, the esterification reaction is completed, and then the benzene is recovered, cooled, washed with alkali, washed with water, dried, and distilled under reduced pressure to obtain the finished product. 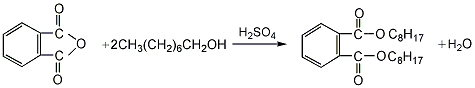
2. Production of ester from phthalic anhydride and n-octanol under reduced pressure It is divided into two types: batch method and continuous method.
(1) The intermittent method is suitable for small batch production. The material ratio is phthalic anhydride: octanol = 1:2 (mass ratio), sulfuric acid is 0.25% to 0.3% of the total materials, and the activated carbon added during esterification is 0.1% to 0.3% of the total materials. The esterification temperature is 150°C, the vacuum degree during esterification is about 0.093MPa, and the esterification time is about 3 hours. The hot water temperature for crude ester washing is 80 to 85°C. The temperature for distillation to recover octanol is 130~140℃, and the vacuum degree is not less than 0.093MPa. If the finished product is required to be of high quality, the crude ester after dealcoholization needs to be distilled and then filtered.
(2) The continuous method is suitable for large-scale production. First, monoesterification is performed. The material ratio is phthalic anhydride: octanol = 1:1.6 (mass ratio). The amount of sulfuric acid is 0.5% of the total amount. The reaction temperature is 120°C. The monoesterification reaction generates a transparent and uniform liquid. The second step of esterification is carried out in the tower. The monoester liquid continuously enters the tower and is continuously esterified at 130-150°C and a vacuum greater than 0.093MPa. The crude ester is washed with 1/5 volume of 50°C water, and continuously neutralized with 2% to 3% soda ash at 60 to 70°C; the neutralized crude ester liquid is washed with 1:1 volume of 70 to 80°C water. of water for washing. Then add 0.1% activated carbon and continuously dealcoholize at a vacuum of 0.097MPa and a temperature of 150°C. After dealcoholization, the ester liquid is filtered by pressure at 100-120°C to obtain the finished product.
Refining method: Contains impurities such as free acid, alcohol, and monooctyl phthalate. During refining, wash with dilute sodium hydroxide solution, dry with anhydrous potassium carbonate or sodium sulfate, and then distill under reduced pressure.
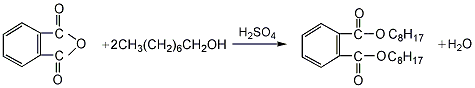
3.P-toluenesulfonic acid catalytic method: Add 0.05mol of phthalic acid, 0.15mol of n-octanol, and 1g of p-toluenesulfon to a three-necked flask equipped with a thermometer, water separator, and reflux condenser. Acid and 0.1g activated carbon, mix evenly, add n-octanol into the water separator until it is level with the branch pipe opening, heat it with an electronic temperature-adjusting electric heating mantle, after the phthalic acid solid disappears, quickly raise the temperature to 160°C to control the reaction temperature At 160~180℃, reflux and separate water for 1 hour. After the reaction is completed, cool the reaction solution to room temperature, wash it with an equal volume of saturated brine 2 to 3 times to make it neutral, add a small amount of activated carbon to the crude product, and depressurize the water pump first, and then distilled under reduced pressure using an oil pump to collect the fraction at 224-234°C to obtain a light yellow, transparent and slightly odorous oily liquid.
Purpose
1. Used as plasticizer. The plasticizing efficiency is the same as that of DOP, while the cold resistance, volatility resistance and stability to the viscosity of plasticized paste are better than DOP, but the insulation is slightly worse. Can be used as polyvinyl chloride, vinyl chloride-vinyl acetate copolymer, polyvinyl butyral, butyl cellulose acetate, nitrocellulose, ethyl cellulose, polymethyl methacrylate, polystyrene and other resins and plasticizers for synthetic rubber. It can be used to make food packaging materials and medical and sanitary products. This product can also be used as a solvent and gas chromatography stationary solution.
2. Mainly used as a plasticizer for polymer materials. The plasticizing efficiency is the same as DOP, while the cold resistance, weather resistance and volatility resistance are better than DOP, but the price is higher and the application is limited. Can be used for PVC, resin, synthetic rubber, etc.
3. Gas chromatography stationary solution, selectively retains and separates aromatic compounds, unsaturated compounds and various oxygen-containing compounds (alcohols, aldehydes, ketones, esters, etc.). Solvent. Plasticizer.

 微信扫一扫打赏
微信扫一扫打赏

