Background and overview[1]
Diphenylthiocarbazine is a hydrazine derivative, which can be used as a pharmaceutical synthesis intermediate. For example, dithizone (dithizone), whose scientific name is diphenylcarbazone, is a blue-black crystalline powder. It is mixed with an aqueous solution of metal and shaken to generate a metal complex salt in the aqueous phase, which is then transferred to the organic The solvent layer undergoes significant color changes. According to the color and depth of the metal complex salt, it can be used for the determination of certain trace metals, such as mercury, lead, zinc, cadmium, etc., especially for trace amounts of lead, it has extremely high sensitivity, good stability and sharpness. Color change is an important analytical reagent in analytical chemistry science.
Structure

Preparation method[1]
The preparation of diphenylthiocarbazine is as follows:
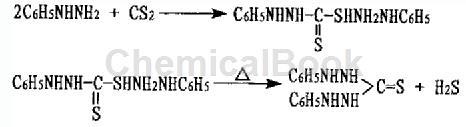
The operation method of step 1) is as follows: add carbon disulfide dropwise to the ethanol solution of phenylhydrazine under constant stirring. The weight ratio of phenylhydrazine to ethanol is 1:4~4.5, and the molar ratio of phenylhydrazine to carbon disulfide is 1 ∶0.55~0.70, reaction temperature 20~25℃, dropwise addition time of carbon disulfide is 3~3.5 hours, continue stirring for 25~35 minutes after the dropwise addition, the generated β-phenyl-dithiocarbazinic acid phenylhydrazine salt remains In the container.
The operation method of step 2) is: reflux and heat the β-phenyl-dithiocarbazinic acid phenylhydrazine salt obtained in step 1) to release hydrogen sulfide gas and finally ammonia gas. The reaction temperature is 75-78°C. Control the end point in the middle, cool, discharge, and centrifuge to dryness to obtain the intermediate diphenylthiocarbazine.
Main reference materials
[1] (CN1970537) Manufacturing method of dithizone

 微信扫一扫打赏
微信扫一扫打赏

