Background and overview[1]
Heterocyclic compounds are widely found in nature, accounting for about half of organic compounds. 2-Aminothiazole ring derivatives are a very important member of heterocyclic compounds. 2-Amino-4-(3-bromophenyl)thiazole can be used as a pharmaceutical synthesis intermediate. This structure is widely used in the fields of pesticides and medicine. Many natural products or small molecule drugs with anti-inflammatory, anti-tumor, anti-viral, and sedative effects contain 2-aminothiazole rings, which have become one of the most commonly constructed functional groups in green drug research in recent years. one.
So far, a variety of construction methods for 2-aminothiazole ring functional groups have been reported, the most classic of which is prepared by refluxing 2-bromoacetophenone with thiourea in ethanol. In addition, benzene Formyl isothiocyanates, secondary amines, and butynyl esters use enzyme-catalyzed one-pot methods to construct this functional group, as well as methods such as nanoparticles and iodine-catalyzed conversion of acetophenones into thiazole rings. However, these methods generally have complex operations and reaction problems. Disadvantages include severe conditions and difficulty in control. Some studies have developed a method for synthesizing 2-aminothiazole ring compounds from ethylbenzene compounds with high yield, mild reaction, low cost and environmental friendliness.
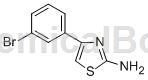
Preparation[1]
The preparation of 2-amino-4-(3-bromophenyl)thiazole is as follows: add 3mL water, 30μL cyclodextrin, 0.50mmol 1-bromo-3-ethylbenzene, 1.50mmol NBS and 0.05mmol AIBN, react at 60°C for 4 hours, cool after the reaction is completed, then add 1.50mmol sodium bicarbonate and 0.50mmol thiourea in sequence, react at 80°C for 1 hour, add ethyl acetate after the reaction, add saturated brine for extraction, and concentrate the organic Phase, column chromatography obtained 97 mg of white solid 2-amino-4-(3-bromophenyl)thiazole, with a yield of 76%.
Product characterization: 1HNMR (CDCl3, 600MHz) δ: 7.97 (s, 1H), 7.72 (d, J=7.5Hz, 1H), 7.44 (d, J=7.7Hz, 1H), 7.3-7.26 (m , 1H), 6.78(s, 1H), 5.20(s, 2H).
Main reference materials
[1] (CN108863978) A method for synthesizing 2-aminothiazole ring compounds from ethylbenzene compounds

 微信扫一扫打赏
微信扫一扫打赏

