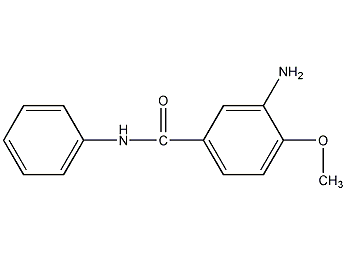
Structural formula
| Business number | 03C1 |
|---|---|
| Molecular formula | C14H14N2O2 |
| Molecular weight | 242.28 |
| label |
3-amino-4-methoxy-N-phenylbenzamide, 4-methoxy-3-aminobenzoanilide, 3-Amino-4-methoxy-n-phenyl-benzamid, 3-Amino-p-anisanilide, Fragrance |
Numbering system
CAS number:120-35-4
MDL number:MFCD00017166
EINECS number:204-388-4
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Characteristics: White ( or light yellow) Powder.
2. Density (g/mL,20℃): Undetermined
3. Relative Vapor Density (g/mL,Air =1): Undetermined
4. Melting point (ºC):154- 156
5. Boiling point (ºC,normal pressure): Undetermined
6. Boiling point (ºC,20mmHg): Undetermined
7. Refractive index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,25ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of the partition coefficient for water: undetermined
17. Isotonic specific volume (90.2K):529.3
4. Surface Tension (dyne/cm):54.7
5. Polarizability(10-24cm3):28.44
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecule polar surface area 64.4
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 277
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides.
Storage method
Save sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep away from sources of fire and store away from oxidizing agents.
Synthesis method
Method 1: Common in current production The method adopted. Using o-nitroanisole as raw material, the product is obtained through chloromethylation, hydrolysis, oxidation, acid chlorination, condensation and reduction. .
This method has a long process route, long production cycle, and low product yield.
Method 2: This is the latest research method. Using p-chlorobenzoic acid as raw material, the product is obtained through methoxylation, nitration, condensation and reduction. This method has a short process route, high product yield and low cost. .
Add 46.8g p-chlorobenzoic acid, 250mL Methanol and 12g Auxiliary TR-300, reflux 10h, Obtain p-methoxybenzoic acid 41.5g. Add it to the nitrifier, and at the same time add 166g dichloroethane, 0.5g sulfuric acid (98%), in 45℃Add dropwise 19g Nitric acid (98%), complete the addition within 3h , and continue the insulation reaction 2h, the nitrification product 52.7g was obtained. Add it to the condensator, and at the same time add 200mL solvent, 40mL aniline, and raise the temperature to ( 98±2)℃, add dropwise 30mL thionyl chloride (Complete adding ) within 30min and continue the insulation reaction 4h, the condensation product 70.0g was obtained. Add it to the reducer, and at the same time add 350mL sodium hydrosulfide solution (30%), and raise the temperature to (80±2)℃Reaction 15h to obtain the final product 51.8g.
Purpose
Mainly used for dyeing and printing cotton fabrics. It is also used for dyeing viscose fiber and linen. The color is slightly darker and the fastness is good. Can be used as an intermediate for organic pigments. Used as intermediates in organic synthesis.
SPAN lang=EN-US> TR-300, reflux 10h to obtain p-methoxybenzoic acid 41.5g. Add it to the nitrifier, and at the same time add 166g dichloroethane, 0.5g sulfuric acid (98%), in 45℃Add dropwise 19g Nitric acid (98%), complete the addition within 3h , and continue the insulation reaction 2h, the nitrification product 52.7g was obtained. Add it to the condensator, and at the same time add 200mL solvent, 40mL aniline, and raise the temperature to ( 98±2)℃, add dropwise 30mL thionyl chloride (Complete adding ) within 30min and continue the insulation reaction 4h, the condensation product 70.0g was obtained. Add it to the reducer, and at the same time add 350mL sodium hydrosulfide solution (30%), and raise the temperature to (80±2)℃Reaction 15h to obtain the final product 51.8g.
Purpose
Mainly used for dyeing and printing cotton fabrics. It is also used for dyeing viscose fiber and linen. The color is slightly darker and the fastness is good. Can be used as an intermediate for organic pigments. Used as intermediates in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

