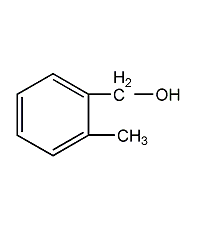
Structural formula
| Business number | 021M |
|---|---|
| Molecular formula | C8H10O |
| Molecular weight | 122.16 |
| label |
2-Methylbenzyl alcohol, 2-methylbenzyl alcohol, 2-Methylbenzyl alcohol, CH3C6H4CH2OH |
Numbering system
CAS number:89-95-2
MDL number:MFCD00004622
EINECS number:201-954-2
RTECS number:None
BRN number:1929783
PubChem number:24851163
Physical property data
1. Melting point (ºC): 37
2. Boiling point (ºC): 223750
3. Relative density (25℃, 4℃): 1.02340
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 37.52
2. Molar volume (cm3/mol): 119.5
3. Isotonic specific volume (90.2K ): 298.4
4. Surface tension (dyne/cm): 38.8
5. Polarizability (10-24cm3): 14.87
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 80.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
1. Preparation method:
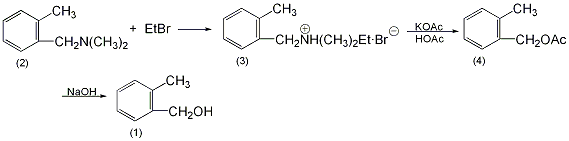
O-methylbenzyldimethylethylammonium bromide (3): In a reaction bottle equipped with a stirrer and a reflux condenser, add 29.8g of o-methylbenzyldimethylamine (2) and 32.7g of bromoethane (0.3mol), 40mL of absolute ethanol, heated to reflux for 1h. Add 10.8g (0.1mol) of ethyl bromide and continue the reflux reaction for 3 hours. The ethanol and unreacted bromoethane were distilled off under reduced pressure. Add 300 mL of anhydrous ether to the remainder, filter the precipitated solid, wash the filter cake with ether, and dry under vacuum to obtain 47.5-49 g of compound (3) with a yield of 92%-95%. O-methylbenzyl acetate (4): In a reaction bottle equipped with a stirrer, add 38.7g (0.15mol) of compound (3), 24.6g (0.3mol) of molten sodium acetate, and 100mL of glacial acetic acid, and heat to reflux for 24 hours. . Cool to room temperature, pour 250 mL of water, and neutralize with 84 g of sodium bicarbonate. Extract with ether three times, combine the ether layers, wash with saturated sodium bicarbonate solution and saturated brine in sequence, and dry over anhydrous magnesium sulfate. Recover ether and then distill under reduced pressure to collect 119~121℃/2.0kPa or 129~131℃/2.8kPaFraction, 21.6~22.4g of o-methylbenzyl acetate (4) was obtained, with a yield of 88%~91%. O-methylbenzyl alcohol (1): Add 50 mL of water, 5 g of sodium hydroxide (0.12 mol), 16.4 g (0.1 mol) of the above compound (4), and 50 mL of methanol into the reaction bottle, and heat to reflux for 2 hours. After cooling, add 50 mL of water and extract with ether three times. The ether layers were combined, washed with water, washed with saturated brine, and dried over anhydrous sodium sulfate. After recovering the ether, add 50 mL of petroleum ether and freeze in an ice-salt bath. The precipitated solid was suction-filtered, washed with cold petroleum ether, and dried to obtain 11.6 to 11.8 g of o-methylbenzyl alcohol (1), with a yield of 95% to 97%. [1]
Purpose
None

 微信扫一扫打赏
微信扫一扫打赏

