Background and overview[1][2]
3,4-Dimethoxyphenylacetic acid, also known as homoveratrol acid, can be used to synthesize the beta-blocker drug bevantolol and the antiarrhythmic drug verapamil, etc. It is also a key intermediate in the synthesis of isoquinoline alkaloids.
Preparation method[1]
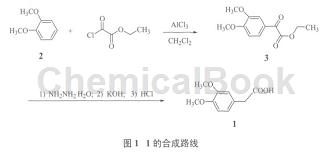
Preparation of ethyl 3,4-dimethoxyacetophenonate (3):
Add anhydrous aluminum trichloride (14.7 g, 0.11 mol), methylene chloride (110 ml) and 2 (13.8 g, 0.10 mol) into a 250 ml neck flask equipped with an electric stirring and exhaust gas absorption device, stir After homogenization, slowly add oxalyl chloride monoethyl ester (15.0 g, 0.11 mol) dropwise in an ice bath, and complete the dropwise addition within about 30 minutes. Remove the ice bath, react at room temperature for 6 hours, and then heat to about 35°C for 4 hours. After the reaction, cool in an ice-water bath, add water (100 ml), and separate the organic layer. The aqueous layer was extracted with dichloromethane (30 ml×2), and the organic layers were combined. Wash with 5% sodium bicarbonate aqueous solution (50 ml Yellow solid 3 (20.8 g, 87.3%), mp 35~36°C. 1H NMR(CDCl3, 400 MHz)δ: 7.64(d,J=8.4 Hz, 1H, ArH), 7.57(s, 1H, ArH), 6.93 (d, J=8.8 Hz, 1H, ArH), 4.45(q, J=7.2 Hz, 2H, CH2CH3), 3.98(s, 3H, OCH3), 3.95(s, 3H, OCH3), 1.43(t, J =7.2 Hz, 3H, CH2CH3).
Preparation of 3,4-dimethoxyphenylacetic acid (1):
Add 3 (17.9 g, 0.075 mol), ethylene glycol (60 ml) and 85% hydrazine hydrate (22.1 g, 0.375 mol) into a 250 ml three-neck flask, and slowly raise the temperature to 120°C for 2 h. After cooling to room temperature, add hydrogen
Potassium oxide (12.6 g, 0.225 mol), heated to 180°C, evaporated the low boiling matter while raising the temperature (about 2 h), and then refluxed for 4 h. After the reaction is completed, cool to room temperature and add 6 mol/L hydrochloric acid (about 30 ml) to adjust the pH to 2~3. Add water (150 ml), extract with ethyl acetate (50 ml×4), and combine the organic phases. After washing with water (50ml g, 73.4%), mp 95~96 ℃ (literature: 96.2~97.5 ℃). 1H NMR(DMSO-d6, 400 MHz)δ:12.23(br s, 1H, OH), 6.87(d, J=8.4 Hz, 1H, ArH),6.85(s, 1H, ArH), 6.76(d, J=8.0 Hz, 1H, ArH), 3.72(s, 6H, OCH3), 3.48(s, 2H, CH2).
Apply[2]
It can be used to prepare homoveratrate:
CTAB surfactant was stirred in 20 ml of water at 40°C for 1 hour until clear, and 12.5g NaSiO·9HO was dissolved in 10 ml of water and stirred at 40°C until clear; both were cooled to room temperature, and the sodium silicate solution was slowly added dropwise to Mix in CTAB solution and stir for 15 min. Then adjust the pH to 10.5 with 2mol/L hydrochloric acid, continue stirring for 3 hours at 25°C, transfer the uniform gel to a polytetrafluoroethylene crystallization kettle for crystallization at 105°C, and then filter and wash with deionized water. Dry in an oven at 105°C for 24h, and finally calcine in a muffle furnace at 550°C for 8h to obtain the traditional hydrothermal method acidic molecular sieve MCM-41.
Weigh 2g (0.01mol) of homoveratrol acid, measure a certain amount of alcohol (0.3mol) and add it to a 75ml high-pressure reaction kettle, add 0.05g of the traditional hydrothermal molecular sieve MCM-41 of Comparative Example 1, and put in the magnet , nitrogen was replaced three times, 2.5Mpa nitrogen was charged, the rotation speed was 630/min, and the reaction was carried out at 100°C for 4 hours. The reaction product was centrifuged to collect the supernatant to obtain homoveratrate, which was used as a comparison group; when the reactant was methanol, the reaction was The conversion rate of veratonic acid is 8.28%, and when the reactant is ethanol, the conversion rate of homoverteric acid is 12.53%.
Main reference materials
[1] Yao Guoxin, Chen Gang, Lu Yong, Zhu Jintao. New synthesis process of 3,4-dimethoxyphenylacetic acid [J]. Chinese Journal of Pharmaceutical Industry, 2010, 41(10): 730-731.
[2] CN201810959715.8 A method for preparing acidic mesoporous molecular sieve using sodium persulfate and application of acidic mesoporous molecular sieve

 微信扫一扫打赏
微信扫一扫打赏

