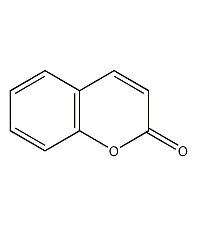
Structural formula
| Business number | 023V |
|---|---|
| Molecular formula | C9H6O2 |
| Molecular weight | 146.14 |
| label |
coumarin, coumarin, oxanaphthone, o-Hydroxycinnamolactone, 1,2-benzopyrone, o-Hydroxycinnamic acid lactone, 5,6-benzo-a-pyrone, Coumarin, Oxygen phthalazinon adjacent ketone, Salicylic acid lactone, 1,2-Benzopyrone, 1-Benzopyran-2-one, o-Hydroxycinamic acid lactone, (Z)-o-Coumarinic acid lactone, spices, Heterocyclic compounds |
Numbering system
CAS number:91-64-5
MDL number:MFCD00006850
EINECS number:202-086-7
RTECS number:GN4200000
BRN number:383644
PubChem number:24892679
Physical property data
1. Properties: Colorless flake or powdery crystals, with a hay aroma similar to black tonka beans.
2. Density (g/mL, 20/4℃): 0.935
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 69~71
5. Boiling point (ºC, normal pressure): 290~301, 154ºC (1.33kpa)
6. Boiling point (ºC, normal pressure): 290~301, 154ºC (1.33kpa)
6. ºC, 1.3kPa): 154
7. Boiling point (ºC, 0.67kPa): 139
8. Flash point (ºC): 162
9. Specific rotation (º): Undetermined
10. Sublimation temperature (ºC): 100
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Solubility (g/100mL, 25ºC, Water): 0.25
15. Solubility (g/100mL, 100ºC, water): 2.0
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Insoluble in cold water, easily soluble in hot water, alcohol, ether, chloroform and sodium hydroxide solution.
Toxicological data
1. Acute toxicity: oral administration – rat LD50: 293 mg/kg; oral administration – mouse LD50: 196 mg/kg.
2. The maximum dosage in food is 5mg/kg food; in alcoholic beverages it is 10mg/kg beverage. The U.S. Bureau of Alcohol, Tobacco, and Firearms (BATF) stipulated in 1974 that the coumarin content in alcohol should not exceed 5 mg/kg.
Ecological data
None
Molecular structure data
1. Molar refractive index: 39.76
2. Molar volume (cm3/mol): 117.0
3. Isotonic specific volume (90.2K ): 305.6
4. Surface tension (dyne/cm): 46.4
5. Polarizability (10-24cm3): 15.76
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 196
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Coumarin is a lactone of cis-ortho-hydroxycinnamic acid. Reactions such as halogenation, nitration, sulfonation, and hydrogenation can occur.
2.Toxic, rat oral LD50293mg/kg, mouse oral LD50196mg/kg, mice intraperitoneal injection LD50220mg/ kg. The workplace should have good ventilation, equipment should be sealed, and operators should wear protective equipment.
3. Found in burley tobacco leaves.
4. Naturally found in black tonka beans, vanilla beans, lavender oil, cinnamon oil, citronella oil, Peru balsam middle.
Storage method
1. This product should be sealed and stored in a cool, dry place away from light.
Packaged in 2.50kg cardboard drum lined with plastic bags. During storage and transportation, attention should be paid to dryness, ventilation, sun protection, moisture-proof, and heat insulation.
Synthesis method
1. Prepared from salicylaldehyde via Perkin reaction. This was the synthesis method originally used by Perkin in 1868. Calcined potassium carbonate can be used as a catalyst for the reaction of salicylaldehyde and acetic anhydride. Add carbonic acid and acetic anhydride to salicylaldehyde, heat to 187°C, evaporate acetic acid when the gas phase temperature is about 120°C, then add acetic anhydride, and react at 210-212°C. The obtained crude coumarin product is washed with water and then vacuum distilled. Then recrystallize with ethanol or isopropyl alcohol and dry at 50°C to obtain the finished product.
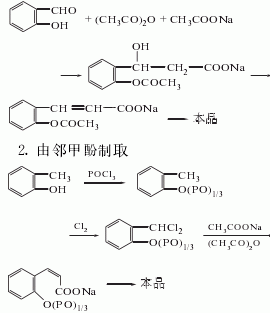
By o-cresol and phosphorus oxychloride After the reaction, the intermediate o-cresol phosphate is obtained, which is chlorinated at 180°C to change the methyl group into -CHCI2, and then reacted with sodium acetate at 160-180°C for 5 hours to perform cyclization. If o-cresol interacts with phosgene, the process is completed through the intermediate o-cresol carbonate: o-cresol sodium salt reacts with phosgene at 60°C and 0.15MPa to generate o-cresol carbonate. Chlorine the esterification with chlorine gas at 180-185°C until the chlorine content is about 37%, which is equivalent to two chlorine atoms on each cresol side chain. Finally, in the presence of pyridine or zinc oxide and cobalt oxide, it is condensed with acetic acid and acetic anhydride to obtain coumarin. The yield of this method is quite high.
3. Tobacco: BU, 14.
Purpose
It is used as a flavoring agent for rubber and plastic products, and is also used in flavors for food, tobacco and wine. It can also be used as a polishing agent and brightening agent for metal surface processing, and as an intermediate and drug in the pharmaceutical industry.

 微信扫一扫打赏
微信扫一扫打赏

