Background and overview[1][2]
4-Fluorobenzoylformaldehyde hydrate is an aldehyde derivative that can be used as a pharmaceutical synthesis intermediate. If 4-fluorobenzoylformaldehyde hydrate is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable.
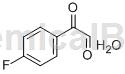
Structure
Preparation method[1]
4-Fluorobenzoylformaldehyde hydrate is prepared as follows:
Method 1: Add 14 grams of selenium dioxide, 62 ml of dioxane and 2.5 ml of water into a 100 ml round-bottomed flask, heat and stir at 50°C until the selenium dioxide dissolves, then add 15 grams of 4-chloroacetophenone After refluxing for 4 hours, the reaction solution was cooled, filtered, and the solvent was removed under reduced pressure. The residue was subjected to column chromatography (ethyl acetate: petroleum ether = 1:5) to obtain colorless oily liquid 4-fluorobenzoylformaldehyde hydrate. 1H‑NMR (300Hz, CDCl3‑d6) δ: 8.06 (d, J=9.0Hz, 2H), 7.46 (d, J=9.0Hz, 2H), 6.29 (s, 1H).
Method 2: Add selenium dioxide (6.70g, 61.0mmol) to a solution of 4-fluoroacetophenone (8.43g, 61.0mmol) in dioxane (100mL) and water (3mL), The resulting mixture was heated at 55°C until complete dissolution of the selenium dioxide occurred. Then, the reactants were refluxed for 5-6 hrs. After the reaction was complete (TLC), the mixture was filtered and the filtrate was concentrated in vacuo to give a viscous oil. Water (50 mL) was added and the resulting mixture was stirred for 12 hrs, after which the solid was collected on the filter, washed with water (25 mL), and dried in vacuo to obtain 4-fluorobenzoylcarboxaldehyde hydrate (6.60 g, 85%). :
1HNMR: (400MHz, CDCl3) δ: 7.18 (m, 2H), 8.11 (d, 2H), no aldehyde CHO signal was observed.
Main reference materials
[1]CN201010579221.0[1,2,4]triazolo[4,3-b][1,2,4]triazine compounds, their preparation methods and uses
[2] CN201180017840.31, 2,4-triazine-4-amine derivative

 微信扫一扫打赏
微信扫一扫打赏

