
Structural formula
| Business number | 059P |
|---|---|
| Molecular formula | CH8O3N2 |
| Molecular weight | 96.09 |
| label |
ammonium bicarbonate, Hartshorn salt, Glucitol, Extinguishing agent, detergent, buffer, neutralizer, leavening agent, accelerator |
Numbering system
CAS number:506-87-6
MDL number:MFCD00010890
EINECS number:208-058-0
RTECS number:BP1925000
BRN number:4253487
PubChem number:24891462
Physical property data
1. Physical property data
1. Properties: colorless, translucent, hard crystalline block or powder. There is a strong ammonia odor. It tastes spicy. It is a mixture of ammonium bicarbonate (NH4HCO3) and ammonium carbamate (NH2COONH4) . Irritating.
2. Density (g/mL, 25/4℃): 2.2
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): Uncertain
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (ºC): Uncertain
10. Autoignition point or ignition temperature (ºC) is uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V /V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Soluble in water,Insoluble in ethanol, carbon disulfide and concentrated ammonia.
Toxicological data
Acute toxicity: Mouse intravenous LD50: 96mg/kg, convulsions or epilepsy, respiratory irritation;
Dog intravenous LDLo: 200mg/kg, no details except lethal dose;
Rabbit subcutaneous LDLo: 900mg/kg, no detailed instructions except the lethal dose;
Rabbit intravenous LDLo: 200mg/kg, no detailed instructions except the lethal dose;
Rabbit transrectal LDLo: 800mg/kg���No details except lethal dose;
Frog subcutaneous LDLo: 250mg/kg, no details except lethal dose;
Frog parenteral LDLo: 758mg/kg , no detailed description except the lethal dose;
After contact, it will irritate the nose, pharynx, and lungs, and may cause coughing and difficulty breathing.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 58.5
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 26.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters : 0
14. The number of uncertain stereocenters of chemical bonds: 0
15. The number of covalent bond units: 2
Properties and stability
1. It is unstable in the air and will gradually turn into ammonium bicarbonate and ammonium carbamate. The dried material decomposes easily at 58°C, releasing ammonia and carbon dioxide. The aqueous solution begins to decompose at 70°C. Unstable to light and heat. Slightly hygroscopic. When placed in the air, it loses ammonia and turns into ammonium bicarbonate. It decomposes to release carbon dioxide at 270°C. 2.Irritating to the skin
Storage method
1. Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed.
2. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Carbonization method: Carbon dioxide, ammonia and steam are directly synthesized into ammonium carbonate, passed into the cooling chamber, directly cooled with water, and then refined to obtain ammonium carbonate finished product. The reaction formula is as follows: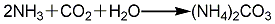
Mix industrial ammonium carbonate and concentrated ammonia water , cooling, translucent crystals first precipitate, and then turn into bright needle-shaped crystals. Quickly suction filter and dry to obtain pure ammonium carbonate.
2. Obtained by adding ammonium sulfate to calcium carbonate solution and heating. 3. Use a mortar to grind the commercial product into powder, put it into a sealable container, slowly add ammonia water to dissolve the powder, and shake thoroughly until the ammonium carbonate is completely dissolved. Place it at 20-25℃ for 2 days, then place it in a cool place at 10-12℃. After a long period of time, crystals will precipitate. Quickly filter, dry and transfer to a completely sealed container. Continue to add 1/3 volume of ethanol to the filtrate, and the crystals will further precipitate. The product thus produced is a monohydrate. 4. Obtained by adding ammonium sulfate to calcium carbonate solution and heating.
Purpose
1. Bit by bit analysis of lithium, radium, thorium and carbonate synthesis, etc. It is also used as fertilizer, fire extinguishing agent, detergent, and in industries such as medicine, rubber, and fermentation.
2. Used as raw materials for baking powder, various ammonium salts, buffers, printing and dyeing auxiliaries, fertilizers and analytical reagents. Edible ammonium carbonate is used as a buffer, neutralizing agent, leavening agent, and fermentation accelerator (for making wine).
3. As buffering agent and neutralizing agent , leavening agents and fermentation accelerators (for making wine). EEC is mainly used to prepare baking powder.
4.Ammonium carbonate is used in sulfosalicylic acid silver plating electrolyte, but the heavy metals and sulfur in it need to be strictly controlled. and the content of thiocyanate, otherwise the anode will easily turn black. In addition, ammonium carbonate is also used in solutions for removing cadmium, nickel and other coatings.

 微信扫一扫打赏
微信扫一扫打赏

