Background and overview[1]
2,4-Dichlorobenzamide is a pharmaceutical intermediate that can be prepared from 2,4-dichlorobenzoic acid.
Preparation[1]
Method 1. Preparation of A. 2,4-dichlorobenzoyl chloride
In a 1000mL single-neck bottle, dissolve 2,4-dichlorobenzoic acid (100.0g, 523.5mmol) in dry tetrahydrofuran (200mL), add 4 drops of dry N,N-dimethylformamide, and chlorine Sulfoxide (93.4g, 785.3mmol). Reflux reaction for 5 hours. After the reaction is completed, the reaction solution is concentrated under reduced pressure and set aside for use.
B. Preparation of 2,4-dichlorobenzamide
In a 1000mL three-necked flask, add ammonia water (500mL), cool to -10°C, add the concentrated solution dropwise, keep warm for 0.5h, and react at room temperature for 1h. After the reaction was completed, the reaction was suction filtered, and the filter cake was vacuum dried to obtain 95.0 g of 2,4-dichlorobenzamide, with a yield of 95.5%.
Apply[1]
Can be used to prepare halogenated isophthalonitrile, which is an important chemical and pharmaceutical intermediate.
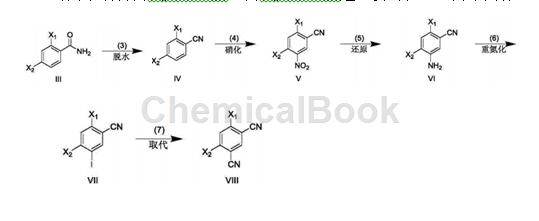
A. Preparation of 2,4-dichlorobenzonitrile.
In a 1000mL three-necked flask, dissolve 2,4-dichlorobenzamide (95.0g, 500.1mmol) in dry N,N-dimethylformamide (280mL), and cool to -15°C. Slowly add phosphorus oxychloride (383.4g, 2500.4mmol) dropwise, keep warm for 0.5h, and react at room temperature for 7h. After the reaction is completed, the reaction solution is slowly poured into ice and a solid is precipitated, and 71.1g of 2,4-dichlorobenzonitrile is obtained by suction filtration with a yield of 82.6%.
B. Preparation of 2,4-dichloro-5-nitrobenzonitrile
In a 1000mL three-necked flask, dissolve 2,4-dichlorobenzonitrile (71.1g, 413.2mmol) in concentrated sulfuric acid (110mL), cool to -10°C, and add fuming nitric acid (169.2g, 2685.9mmol) dropwise ), incubate for 0.5h, and react at room temperature for 4h. After the reaction is completed, pour the reaction solution slowly into ice and solid will precipitate, filter with suction, and vacuum-dry the filter cake to obtain 75.6g of 2,4-dichloro-5-nitrobenzonitrile, with a yield of 84.3%.
C. Preparation of 5-amino-2,4-dichlorobenzonitrile
In a 1000mL single-neck bottle, dissolve 2,4-dichloro-5-nitrobenzonitrile (75.6g, 348.5mmol) in absolute ethanol (500mL), add glacial acetic acid (125.6g, 2091.0mmol), Add reducing iron powder (58.4g, 1045.5mmol) in batches, react at room temperature for 1 hour, and at 50°C for 4 hours. After the reaction is completed, filter with suction, wash the filter cake with ethyl acetate (80ml×5), collect the filtrate, extract with ethyl acetate (200ml×5), combine the organic phases, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and use A small amount of n-hexane (30 mL) was used for pulping, and then suction filtration was performed to obtain 53.8 g of 5-amino-2,4-dichlorobenzonitrile, with a yield of 82.5%.
D. Preparation of 2,4-dichloro-5-iodobenzonitrile
In a 5000mL three-necked flask, cool 5-amino-2,4-dichlorobenzonitrile (53.8g, 287.6mmol) to -10°C, and add 1mol/L sodium nitrite (29.8g, 431.4 mmol) aqueous solution, incubate for 2 h, add dropwise 0.5 mol/L potassium iodide (71.6g, 431.4 mmol) aqueous solution, and incubate for 4 h. After the reaction is completed, extract with ethyl acetate (200mL×3), combine the organic phases, wash with saturated sodium thiosulfate (150mL×1), dry with anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and beat the residue with a small amount of n-hexane (30mL) , suction filtration yielded 42.9g of 2,4-dichloro-5-iodobenzonitrile, with a yield of 50.1%.
E. Preparation of 4,6-dichloroisophthalonitrile
In a 500mL single-neck bottle, dissolve 2,4-dichloro-5-iodobenzonitrile (42.9g, 144.1mmol) in N,N-dimethylformamide (180ml), and add zinc cyanide (10.2 g, 86.5mmol) and tetrakis(triphenylphosphorus)palladium (9.0g, 7.2mmol), react at room temperature for 0.5h, and at 120°C for 10h. After the reaction is completed, filter with suction while it is hot. Pour the filtrate into ice water and a solid will precipitate. Filter with suction. Slurry the filter cake with a small amount of n-hexane (20 mL). Filter with suction to obtain 20.1g of the target product 4,6-dichloroisophthalonitrile. , the yield is 70.7%. 1H NMR (400MHz, CDCl3): δ7.98 (s, 1H), 7.76 (s, 1H).
Main reference materials
[1] [Chinese invention, Chinese invention authorization] CN201611153520.1 A preparation method of 4,6-bishalogenated isophthalonitrile [Public]/A method of preparing 4,6-bishalogenated isophthalonitrile Preparation method【Authorization】

 微信扫一扫打赏
微信扫一扫打赏

