Overview[1][2]
3-Hydroxyanthranilic hydrochloride is an amino compound and can be used as a pharmaceutical synthesis intermediate.
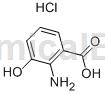
Structure
Preparation[1-2]
Method 1: 3-Hydroxyanthranilic hydrochloride is prepared as follows: Add 6.0g 85.3% H3PO4 to a 100ml three-neck flask equipped with a mechanical stirrer, nitrogen outlet and oil bubbler. Cool to 0°C in an ice bath. To this solution was added 9.72g P2O5 (88% P2O5 content). Heat the viscous mass to 150°C and stir for 6 hours; then add pre-dried 1.7g HAA (2.1mmol) and 1.7g 2-amino-3-hydroxybenzoic acid (2.1mmol). The temperature was raised to 185°C and the reaction was allowed to proceed under positive nitrogen pressure for an additional 18-32 hours. The resulting reaction mixture was precipitated in water, filtered and washed with water in a Soxhlet extractor for 24 hours. The solid was placed in a vacuum oven and dried at 100 °C for 24 h, acidified with hydrochloric acid, and the product was characterized by IR, TGA, and DSC, and the intrinsic viscosity of the material was measured.
Method 2: Use Penicillium catalysis to prepare 3-hydroxyanthranilic hydrochloride. The specific steps are as follows: 15000L fermentation tank, that is, prepare a microbial fermentation liquid fermentation tank, and add the substrate 2-aminobenzoate to the fermentation liquid. Phenol, the concentration is 90-95g/L, add the following ingredients, the concentration is: linear 10-carbon alcohol polyoxyethylene ether is 10-12g/L, xylose 20-24g/L, sucrose 25-28g/L L. Glutinous rice flour 18-22g/L, autoclave at 121°C for 30 minutes; when cooled to 33-35°C, stir and emulsify, and perform biocatalytic reaction. The reaction time is 49-54 hours; when the reaction starts, add the concentration immediately It is 5% NaHCO3 aqueous solution, and at the same time, add 1% hydrochloric acid aqueous solution, adjust the flow acceleration rate of NaHCO3 and hydrochloric acid aqueous solution, and maintain pH 6.4-6.6 , the added materials have been sterilized; the ventilation ratio is 0.6-0.7V/V, that is, the ventilation volume per minute is 0.6-0.7 times the volume of the reaction solution. After the reaction is completed, break the emulsion and extract with a countercurrent extraction machine. It is ethyl acetate, the ethyl acetate in the organic phase is evaporated, and the ethyl acetate is evaporated to obtain the product 2-amino-3-hydroxy-benzoformic acid. The reaction conversion rate is 98.1-99.6%, and the product yield is 95.9-97.1%.
Main reference materials
[1](US5340913)Synthesisofaromaticheterocyclicpolymersfromabiosyntheticallypreparedprecursor
[2] (CN106399408) Method for preparing amino-hydroxyl benzoic acid by adopting microbes

 微信扫一扫打赏
微信扫一扫打赏

