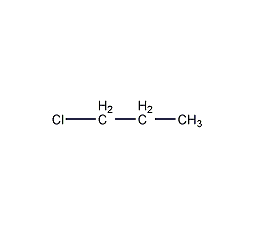
Structural formula
| Business number | 05KA |
|---|---|
| Molecular formula | C3H7Cl |
| Molecular weight | 78 |
| label |
None |
Numbering system
CAS number:540-54-5
MDL number:MFCD00000995
EINECS number:208-749-7
RTECS number:TX4400000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: colorless liquid with chloroform smell. [1]
2. Melting point (℃): -122.8[2]
3. Boiling point (℃): 46.6[3]
4. Relative density (water = 1): 0.89[4]
5. Relative vapor Density (air=1): 2.71[5]
6. Saturated vapor pressure (kPa): 45.9 (25℃)[6]
7. Heat of combustion (kJ/mol): -1999.3[7]
8. Critical temperature (℃): 230[8]
9. Critical pressure (MPa): 4.58[9]
10. Octanol/water partition coefficient: 2.04 [10]
11. Flash point (℃): -32[11]
12. Ignition temperature (℃): 520[12]
13. Explosion upper limit (%): 11.1[13]
14. Explosion lower limit (% ): 2.6[14]
15. Solubility: Slightly soluble in water, soluble in ethanol, ether, benzene and chloroform. [15]
16. Heat of evaporation (KJ/mol, b.p.): 27.26
17. Heat of fusion (KJ/mol): 5.55
18. Heat of formation (KJ/mol, 25ºC, liquid): 159.96
19. Heat of combustion (KJ/mol, 25ºC, liquid): 2022.2
20 .Specific heat capacity (KJ/(kg·K), 25ºC): 1.68
21. Solubility (%, water, 20ºC): 0.271
22. Conductivity (S/m, 20ºC): 5.55×10-4
23. Body expansion coefficient (K-1, 20ºC): 0.001447
24. Relative density (25℃, 4℃): 0.8850
25. Refractive index at room temperature (n25): 1.3858
26. Critical density ( g·cm-3): 0.2965
27. Critical volume (cm3·mol-1): 246.7
28. Critical compression factor: 0.2589
29. Eccentricity factor: 0.228
30. Lennard-Jones parameter (A): 7.975
31.Lennard-Jones parameter (K): 217.1
32. Solubility parameter (J·cm-3)0.5: 17.041
33. van der Waals area (cm2·mol-1): 6.620×109
34. van der Waals volume (cm3·mol-1): 45.750
35. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -161.6
36. Liquid phase standard hot melt (J·mol-1·K– 1): 129.3
37. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -131.9
38. Gas phase standard entropy (J·mol-1·K-1): 318.64
39. Gas phase standard free energy of formation (kJ·mol-1): -52.1
40. Gas phase standard hot melt (J·mol-1·K-1): 84.14
Toxicological data
1. Acute toxicity[16] LD50: >2000mg/kg (rat oral)
2. Irritation No information available
3. AsiaSexual and chronic toxicity[17] Rats inhaled this product at 128.4g/m3, 1 hour a day, 4 days in total. There was mild pulmonary congestion and hepatocellular necrosis.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradable[18] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 15d (theoretical).
Molecular structure data
1. Molar refractive index: 20.79
2. Molar volume (cm3/mol): 89.4
3. Isotonic specific volume (90.2K ): 189.9
4. Surface tension (dyne/cm): 20.3
5. Polarizability (10-24cm3): 8.24
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 7.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: In the presence of aluminum trichloride, isomerization occurs below 200°C and transforms into 2-chloropropane. In the presence of barium chloride, it is heated to 380~400°C to generate propylene and 2-chloropropane.
2. Stability[19] Stable
3. Prohibited use Substances[20] Strong oxidants, strong bases
4. Conditions to avoid contact[ 21] Heating
5. Polymerization hazard[22] No polymerization
6. Decomposition products[23] Hydrogen chloride
Storage method
Storage Precautions[24] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29°C. Keep container tightly sealed. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained from the reaction of n-propanol and hydrochloric acid. Dissolve anhydrous zinc chloride in hydrochloric acid, and slowly add n-propanol while stirring. After the addition is completed, the reaction is refluxed, and the generated chloropropane is evaporated, and all the chloropropane is evaporated to 115-120°C. The steamed crude product is washed with industrial sulfuric acid, water, 10% sodium carbonate, then water, dried with calcium chloride and then fractionated. The 44.6-47.5°C fraction is collected to obtain the finished product. In addition, propyl chloride can also be prepared by reacting n-propanol with phosphorus pentachloride.
Purpose
Used as organic synthesis intermediates and solvents. [25]

 微信扫一扫打赏
微信扫一扫打赏

