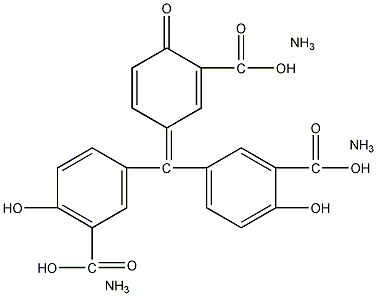
Structural formula
| Business number | 05TT |
|---|---|
| Molecular formula | C22H23N3O9 |
| Molecular weight | 473.44 |
| label |
Rose red ammonium tricarboxylate, salicyl o-aminophenol, Salicylaldehyde o-aminophenol acetate, Golden red ammonium tricarboxylate, Rose red ammonium trihydroxylate, Ammonium aurin trihydroxyate, Aluminum, Ammonium aurintricarboxylate, ATA, Aurintricarboxylic acid ammonium salt, indicator |
Numbering system
CAS number:569-58-4
MDL number:MFCD00040925
EINECS number:209-319-1
RTECS number:GU4800000
BRN number:3900820
PubChem ID:None
Physical property data
1. Physical property data
1. Properties: Brick red crystalline powder. 2. Melting point (℃): 220~2253. Solubility: Easily soluble in water, slightly soluble in ethanol, almost insoluble in ether, acetone and chloroform.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
IV. Calculated chemical data:
2. Number of hydrogen bond donors: 5
3. Number of hydrogen bond acceptors: 9
4. Number of rotatable chemical bonds: 3
5. Topological molecular polar surface area (TPSA): 181
6. Number of heavy atoms: 34
7. Surface charge : 0
8. Complexity: 759
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters: 0
11. The number of uncertain atomic stereocenters: 1
12. The number of determined chemical bond stereocenters: 0
13. The number of uncertain chemical bond stereocenters: 0
14. Number of covalent bond units: 4
Properties and stability
Aqueous solutions are red and neutral, red-purple in alkaline media, and colorless in strong alkalis. With Al3+, Fe3+, In3+ lanthanide elements, UO22 + etc. form red or purple complexes.
Storage method
Keep sealed
Synthesis method
1. Add 10g (0.145mol) solid sodium nitrite in portions to 20ml of concentrated sulfuric acid, while controlling the addition speed so that onlyAmount of nitric oxide. When the solution is completely dissolved, add 20g (0.145mol) salicylic acid in portions while stirring. The whole process takes about 15 minutes. The mixture was then stirred at 20°C until all solids were dissolved and the viscous liquid was bright red to brown. Place the liquid in an ice bath and lower the temperature to 0°C. Slowly add 5ml (0.065mol) of 35-40% formaldehyde solution dropwise while stirring. The reaction temperature should not exceed 5°C. After the reaction is complete, add 100g of crushed ice. and 500ml ice water, stir until solid pieces form. Filter, wash with ice water several times, drain, dissolve in 1:2 (volume ratio) ammonia water, and filter with filter paper. The filtrate is evaporated to dryness on a steam bath. Approximately 19.22g of brown glassy solid was obtained. Yield 83-86%.
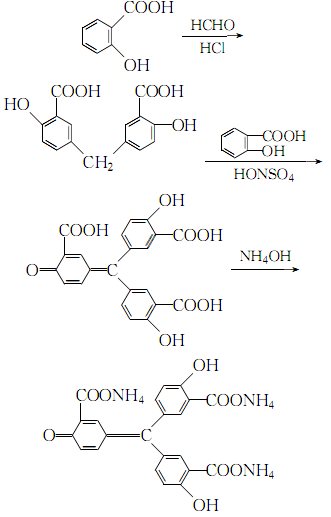
2.Mix salicylic acid and hydrochloric acid evenly, heat to 70℃ with constant stirring, slowly add formaldehyde, and control the temperature not to exceed 80℃ , after the addition, the temperature was raised to 95°C and kept for 8 hours. After the reaction was completed, it was cooled, poured into distilled water, and left to crystallize. The filtered crystals were washed with a large amount of distilled water and dried at 100°C. Mix the obtained methinesalicylic acid and salicylic acid evenly, and add to the nitrosyl sulfuric acid (sodium nitrite solid and 0°C concentrated sulfuric acid quickly) while stirring. Mixed), the temperature is controlled below 15°C. The reaction ends in 20 to 24 hours. The obtained crystals are washed with a large amount of distilled water, boiled with hot water until the crystals are loose, and then washed with distilled water until the washing liquid becomes neutral. Dissolve it with dilute ammonia water, decolorize it with activated carbon, add analytical grade hydrochloric acid to the filtrate, wash the precipitated crystals with water until they are qualified, and dry them to obtain the aluminum reagent. The reaction formula is:
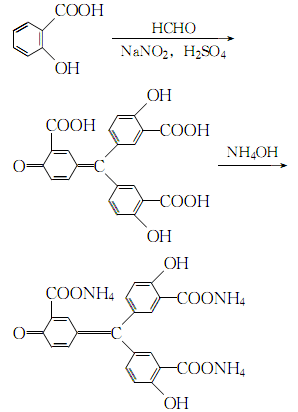
3. Under rapid stirring, add solid sodium nitrite into concentrated sulfuric acid in batches, controlling the addition speed to avoid the production of red smoke. . After it is completely dissolved, add salicylic acid in small amounts in batches, control the temperature not to exceed 20°C, and stir until it is completely dissolved. The mixture will be light red to brown and viscous. Cool the temperature to 0°C, slowly add 37% formaldehyde solution with rapid stirring, and keep the temperature below 5°C. After the reaction is completed, add a large amount of ice-water mixture while stirring until the solid is dispersed. The solid obtained was washed with cold water several times, filtered with suction, dissolved in 1:2 dilute ammonia water, filtered to remove insoluble matter, and evaporated to dryness to obtain yellow ammonium salt. The reaction formula is:
Purpose
1. Complex titration indicator for determination of aluminum in water, food and tissues.
2. Test manganese, gallium and aluminum

 微信扫一扫打赏
微信扫一扫打赏

