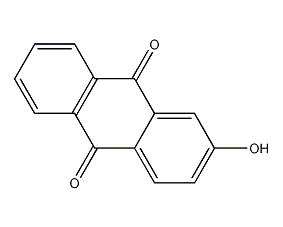
Structural formula
| Business number | 068J |
|---|---|
| Molecular formula | C14H8O3 |
| Molecular weight | 224.21 |
| label |
2-hydroxy-9,10-anthracenedione, 2-Hydroxy-9,10-anthracenedione |
Numbering system
CAS number:605-32-3
MDL number:None
EINECS number:210-085-8
RTECS number:CB7250000
BRN number:None
PubChem ID:None
Physical property data
Physical property data:
1. Character: yellow needle or flake crystal
2. Melting point (℃):306℃
3. Solubility:Soluble in ethanol, ether and hot acetic acid. Insoluble in cold water. Soluble in ammonium hydroxide and alkali to form a reddish-yellow liquid. Soluble in concentrated sulfuric acid to form a reddish-brown liquid
Toxicological data
None yet
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment and should be harmful to water bodies. Give special attention.
Molecular structure data
5. Molecular property data: 1, Molar refractive index:60.55 2, Molar volume (m3/mol):157.4 3, Isotonic specific volume (90.2K):450.0 4, Surface tension (dyne/cm):66.6 5, Polarizability (10-24cm3 ):24.00
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 5
6. Topological molecule polar surface area 54.4
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 349
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Basic properties
Sublimable. Can produce water-soluble barium salts.
Storage method
Storage:
Seal the secret container and store it in a sealed main container in a cool place Dry position.
Synthesis method
2. Introduction to production methods
Mainly composed of anthraquinone-2-Sulfonate or2-Chloranthraquinone is obtained by hydrolysis. It can also be obtained from the roots of the plant Umbelliferae.
Purpose
3. Purpose
For manufacturing2-Methoxy,2-Ethoxy,2-Phenoxyanthraquinone or alizarin and other intermediates, dyes and other fine chemical products.
 微信扫一扫打赏
微信扫一扫打赏

