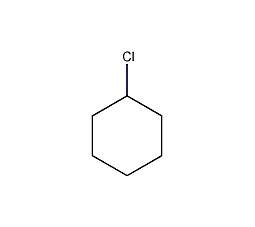
Structural formula
| Business number | 05LD |
|---|---|
| Molecular formula | C6H11Cl |
| Molecular weight | 118 |
| label |
Chlorinated cyclohexane, cyclohexyl chloride, cyclohexyl chloride, Cyclohexane chloride, Chlorocyclohexane, Cyclohexyl chloride, cyclohexylamine chloride, Cyclohexyl chloride, 3-Chloro-1-cyclohexene, Ester cyclic compounds and their derivatives |
Numbering system
CAS number:542-18-7
MDL number:MFCD00003822
EINECS number:208-806-6
RTECS number:GU8685000
BRN number:1900796
PubChem ID:None
Physical property data
1. Properties: colorless liquid
2. Density (g/ cm3, 25/4℃): 1.00
3. Relative Density (20℃, 4℃): 1.000130
4. Melting point (ºC): -43.9
5. Boiling point (ºC, normal pressure) :143
6. Relative density (25℃, 4℃): 0.989430
7. Refractive index: 1.4626
8. Flash point (ºC): 28
9. Refractive index at room temperature (n20): 1.4627
10. Refractive index at room temperature (n25): 1.4607
11. The liquid phase standard claims heat (enthalpy) (kJ·mol-1): -207.2
12. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -163.7
13. Gas phase standard entropy (J·mol-1·K-1): 345.79
14. Gas phase standard free energy of formation (kJ·mol-1): -9.0
15. Gas phase standard hot melt (J·mol-1·K-1): 121.22
16. Oil and water (octanol/ Log value of the distribution coefficient (water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, easily soluble in chloroform. Miscible with ethanol; ether; acetone and benzene.
Toxicological data
1. Acute toxicity: Rats via inhalation LCLo: 40 gm/m3/2H, behavior – excitement, muscle contraction or spasm, difficulty breathing;
Rat LD50 via unknown route: 3gm/kg , no detailed description except the lethal dose;
Mouse inhalation LC50: 31 gm/m3/2H, no detailed description except the lethal dose;
2. Other multiple dose toxicity data : Rat oral TDLo: 4500 mg/kg/26W-I, behavior-changed classical conditioning reflex;
Rat inhaled TCLo: 500 mg/m3/5H/26W-I, blood pressure decreased spontaneously without regularity, white blood cells (white blood cells) Count changes;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 32.58
2. Molar volume (cm3/mol): 120.9
3. Isotonic specific volume (90.2K ): 277.5
4. Surface tension (dyne/cm): 27.7
5. Polarizability (10-24cm3): 12.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 46.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Has a suffocating odor. It decomposes when heated with water, producing hydrochloric acid and turning yellow.
Cyclohexane has anesthetic and skin irritation effects, and its halogenated compounds have stronger effects. The production workshop should be well ventilated and the equipment should be sealed. Operators should wear protective equipment.
Storage method
Stored in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
This product is shipped in iron drums or tank trucks. This product is flammable. It should be stored in a dry, cool and well-ventilated warehouse. Keep away from fire and heat sources. Protect from sun and impact. Store and transport according to regulations for flammable materials. Load and unload gently to prevent packaging damage.
Synthesis method
1. Cyclohexanol chlorination method: obtained by chlorination of cyclohexanol: cyclohexanol and 30% hydrochloric acid are mixed and stirred, heated to 85°C and refluxed for 12 hours, and the temperature gradually rises to about 103°C. After the reflux is completed, cool to 20°C, let stand and separate into layers, remove the acidic water, wash once with saturated sodium chloride solution and sodium carbonate solution, dry with anhydrous calcium chloride, fractionate, collect the 141-142.5°C fraction. Obtain chlorocyclohexane. The yield is 83%.

2. Chlorination of cyclohexane Method: Prepared by chlorination of cyclohexane by passing chlorine gas at 50~90℃.
3. Preparation method:
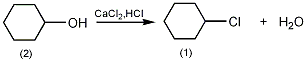
Add 100g (1.0mol) of cyclohexanol (2), 250mL of concentrated sulfuric acid, and 80g of anhydrous calcium chloride into a reaction flask equipped with a stirrer and reflux condenser, and heat in a boiling water bath for 8h with stirring①. Cool and separate the organic layer. The organic layer was washed with saturated brine, saturated sodium bicarbonate, and saturated brine in sequence, dried over anhydrous calcium chloride, and distilled under normal pressure. The fractions at 141.5 to 142.5°C were collected to obtain chlorocyclohexane (1)② 70g, yield 76%. Note: ① Hydrogen chloride gas escapes in large quantities, so be careful to absorb it. ②Prepare chlorocyclopentane from cyclopentanol according to this method, the yield is 57%, bp113~115℃. [1]
4. Preparation method:
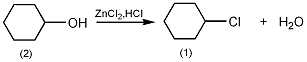
Add 50g (0.5mol) of cyclohexanol (2), 40.8g (0.3mol) of zinc chloride, and 160mL of concentrated hydrochloric acid into the reaction bottle. Heat in a water bath and slowly Slowly increase the temperature to reflux, continue the reaction for 1 hour, cool, and separate the organic layer. The organic layer was washed with saturated brine, saturated sodium bicarbonate, and saturated brine in sequence, dried over anhydrous calcium chloride, and distilled under normal pressure. The fractions at 141-143°C were collected to obtain 51g of chlorocyclohexane (1), yield 86%. [2]
Purpose
Pharmaceutical intermediates, used to prepare anti-epileptic and spasmodic drugs trihexyphenidyl hydrochloride. It is also an intermediate of the pesticide tricyclic tin and a rubber anti-scorch agent.
Tricyclohexyltin chloride is an intermediate used in the synthesis of acaricides tricyclotin and triazoletin. It can also be used to synthesize pharmaceutical trihexyphenidyl hydrochloride, rubber anti-scorch agent and other organic synthetic raw materials, and can also be used as an organic solvent.

 微信扫一扫打赏
微信扫一扫打赏

