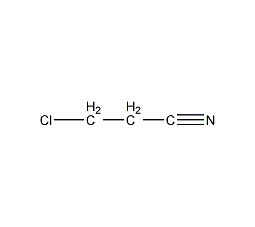
Structural formula
| Business number | 05LR |
|---|---|
| Molecular formula | C3H4NCl |
| Molecular weight | 89.52 |
| label |
β-Chloropropionitrile, Cyanide-beta-chloroethane, Beta-chloropropionitrile, β-Chloropropionitrile, 1-Chloro-2-cyanoethane, Beta-Chloropropionitrile |
Numbering system
CAS number:542-76-7
MDL number:MFCD00001952
EINECS number:208-827-0
RTECS number:UG1400000
BRN number:1098454
PubChem ID:None
Physical property data
1. Properties: Colorless liquid with special odor[1]
2. Melting point (℃): -51[2]
3. Boiling point (decomposition, ℃): 175~176[3]
4. Relative density (water=1): 1.144[4]
5. Relative vapor density (air=1): 3.09[5]
6. Saturated vapor pressure ( kPa): 0.80 (50℃)[6]
7. Octanol/water partition coefficient: 0.18[7]
8. Flash point (℃): 75.6 (CC) [8]
9. Solubility: miscible in ethanol, acetone, ether, benzene, tetrachlorine carbon. [9]
Toxicological data
1. Acute toxicity: rat oral LD50: 10mg/kg, behavior – lethargy (common depressive activity), convulsions or epilepsy, affecting blood vessels – regional or general arterioles or vein dilation;
Mouse oral LD50: 9mg/kg, behavioral – general anesthesia;
Mouse intraperitoneal LD50: 100mg/kg, no details except lethal dose;
Mouse via Intravenous LD50: 56mg/kg, no details except lethal dose;
2. Acute toxicity[10] LD50: 100mg/kg (orally in rats); 9mg/kg (orally in mice)
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 20.71
2. Molar volume (cm3/mol): 82.8
3. Isotonic specific volume (90.2K ): 198.2
4. Surface tension (dyne/cm): 32.8
5. Polarizability (10-24cm3):8.21
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 23.8
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 51.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[11] Stable
2. Incompatible substances [12] Strong acid, strong alkali, strong oxidizing agent, strong reducing agent
3. Conditions to avoid contact[13] Heating
4. Polymerization hazard[14] No polymerization
5. Decomposition products[15] Hydrogen cyanide, hydrogen chloride
Storage method
Storage Precautions[16] Store in a cool, well-ventilated dedicated warehouse. Implement the system of “dual people to send and receive, and double to keep”. Keep away from fire and heat sources. The storage temperature does not exceed 25°C and the relative humidity does not exceed 75%. Keep container tightly sealed. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Originated from the reaction of acrylonitrile and hydrogen chloride. Under cooling, dry hydrogen chloride is passed into acrylonitrile. The hydrogen chloride is quickly absorbed and reacted. Ventilation is stopped when the reaction liquid slowly increases in weight and reaches the theoretical amount. Collect the 68-71°C (2.13kPa) fraction by distillation, wash with 10% sodium carbonate, and dry with anhydrous sodium sulfate. Re-distill the 70-71°C (2.13kPa) fraction to obtain the finished product. The yield is 80%.
Purpose
1. Used for drug and polymer synthesis.
2. Used as an intermediate in organic synthesis. [17]

 微信扫一扫打赏
微信扫一扫打赏

