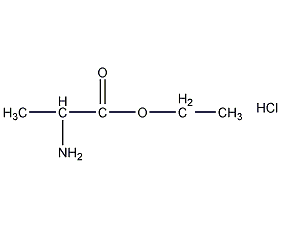
Structural formula
| Business number | 06HV |
|---|---|
| Molecular formula | C5H12ClNO2 |
| Molecular weight | 153.61 |
| label |
Phenyl ethyl hydrochloride, Alanine ethyl ester hydrochloride, DL-alpha-alanine ethyl ester hydrochloride, Hydrochloride-DL-alpha-alanine ethyl ester, Ethyl-α-amino propionate hydrochloride, Racemic alanine ethyl ester hydrochloride |
Numbering system
CAS number:617-27-6
MDL number:MFCD00013018
EINECS number:210-507-0
RTECS number:None
BRN number:3654425
PubChem number:24845824
Physical property data
1. Physical property data
1. Melting point (ºC): 85-87ºC
2. Properties: white crystal.
3. Solubility: soluble in water and ethanol, insoluble in ether
Toxicological data
None yet
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 30.48
2. Molar volume (cm3/mol): 118.6
3. Isotonic specific volume (90.2K): 282.2
4. Surface tension (dyne/cm): 32.0
5. Polarizability (10-24cm3): 12.08
Compute chemical data
IV. Calculated chemical data:
1. Hydrophobic parameter calculation reference value (XlogP): 0
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Topological molecular polar surface area (TPSA): 53.9
6. Number of heavy atoms: 8
7. Surface charge: 1
8. Complexity: 82.5
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters: 1
11. Uncertain number of atomic stereocenters: 0
12. Determine the number of chemical bond stereocenters: 0
13. Number of uncertain stereocenters of chemical bonds: 0
14. Number of covalent bond units: 1
Properties and stability
Properties and stability:
The product may not decompose under normal temperature and pressure.
Storage method
Storage:
Seal the container and store it in a sealed main container in a cool, dry place.
Synthesis method
2. Preparation method: obtained by esterification of α-alanine. Put anhydrous ethanol and α-alanine into the reaction pot, and add dry hydrogen chloride gas at room temperature until it is saturated. After the reaction liquid is completely clarified, raise the temperature back toStreaming 4h. Evaporate excess ethanol to dryness. That is, DL-alanine ethyl ester hydrochloride is obtained.
Purpose
1. Intermediate of vitamin B6.
2. Biochemical research.

 微信扫一扫打赏
微信扫一扫打赏

