Background and overview[1]
Benzothiazole (also known as Besheng or Jiasheng, the full English name is 2-(thiocyanomethylthio)benzothiazole, CAS number is 21564-17-0) is a broad-spectrum seed protection agent , has significant control effect on fungal or bacterial diseases spread by soil and seeds. It can be used for seed dressing, seed soaking, root irrigation or foliar spray to prevent and control rice blast, flax leaf spot, bacterial blight, and sheath blight. Diseases include sugarcane anthracnose, vegetable anthracnose, melon anthracnose, damping-off, blight, vine cutting disease, citrus canker and other diseases. The product name in foreign markets is TCMTB. TCMTB is a fungicide with excellent performance, broad spectrum, high efficiency and low toxicity. The usage abroad is already very large and is rising rapidly. There is no domestic manufacturer yet, mainly because the current process technology makes product production costs high and it is difficult to adapt to the current domestic and foreign markets.
Application
TCMTB is a very economical and effective green fungicide. It belongs to the same category as BUSAN30L from Buckmam Company in the United States and BIOCIDEMT30 from Progiven Company in France. It can effectively Used in seed treatment, it has a strong killing and controlling effect on the main existing bacteria, fungi and algae. It is effective for seed dressing, seed soaking and spraying to treat diseases such as anthrax, rice blast, damping-off, withering and citrus canker. Special effects, therefore, benthiocyanine (benthiocyanine) and its metabolites have large residues in plants and soil.
Preparation[1]
Method 1: 2-mercaptobenzothiazole method
The molecular formula of 2-mercaptobenzothiazole is C7H5NS2, molecular weight 167.25, CAS number 149-30-4. 2-mercaptobenzothiazole is an important variety among thiazole rubber vulcanization accelerators, and it is also an antioxidant , important anti-corrosion additives. Using 2-mercaptobenzothiazole as raw material, it reacts with bromochloromethane so that the hydrogen in -SH on the mercaptobenzothiazole is replaced by chloromethyl to obtain 2-chloromethylthiobenzothiazole. The reaction route is as follows:
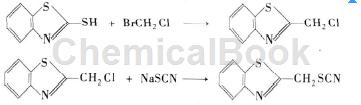
The specific steps are: place bromochloromethane and 2-mercaptobenzothiazole in a three-necked flask, in which the amount of bromochloromethane is about 10 times that of 2-mercaptobenzothiazole. Control the reaction temperature at 25°C and stir for 6 hours. After the reaction is completed, the liquid in the flask is divided into two layers. Use a separatory funnel to separate the lower layer and retain the upper layer. The organic phase was washed several times with water to obtain the crude product of 2-chloromethylthiobenzothiazole, which was treated with n-hexane to obtain the pure product. The pure product obtained was co-heated with sodium thiocyanide and diethylene glycol methyl ether at 70°C. During the reaction, liquid chromatography was used to track the reaction progress. The reaction end point was reached after 5 hours, and the yield was 98%
Method 2: 2-mercaptobenzothiazole improved method
Based on the first method of synthesizing benzothiocyanide, bromochloromethane and sodium thiocyanide are first substituted to generate chloromethyl thiocyanate, and then the thiocyanate is Methyl chloride reacts with 2-mercaptobenzothiazole to form benzothiocyanine (benzothiocyanine). The reaction route is as follows:
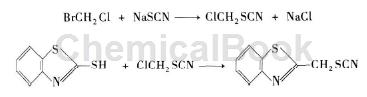
Place bromochloromethane in a three-necked flask, and then add 40% sodium thiocyanate aqueous solution. The material ratio of bromochloromethane to sodium thiocyanate is 1:1. The solution is refluxed at 65 to 77°C. After 14 hours, after the reaction is completed, the organic layer is cooled and separated, washed with water and distilled under reduced pressure. Under a pressure of 13 mmHg, the 71°C fraction is collected to obtain chloromethyl thiocyanate with a yield of 46%. If you want to increase the yield of this reaction, you can add a small amount of phase transfer catalyst. After mixing 2-mercaptobenzothiazole and sodium ethoxide, add a mixed solution of ethanol, isopropyl alcohol, and methanol, use an ice bath to control the reaction temperature below 40°C, add chloromethyl thiocyanate, and keep the reaction temperature at 35~ 40°C, react for 24 hours, leave at room temperature for 15 days, then filter the generated NaCl, wash the filter cake with a mixed solution of ethanol, methanol and isopropyl alcohol, then wash with dichloromethane, collect the filtrate, separate the organic phase, and The layer was dissolved in methylene chloride and then separated by liquid separation. After washing the organic layer with water, it was dried over anhydrous magnesium sulfate to remove water, and then dichloromethane was distilled off to obtain benthiocyanine (benthiocyanine).
Method 3: Chloromethylation route
Using 2-mercaptobenzothiazole as raw material, 2-chloromethylthiobenzothiazole is obtained after chloromethylation, and then according to the second step of the synthesis route of the first method, further obtain benthiocyanine ( benthiocyanate).
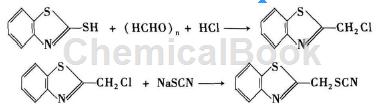
In the chloromethylation reaction, first add dry benzene and 2-mercaptobenzothiazole to the device with a gas introduction tube in sequence, stir at 40°C to dissolve 2-mercaptobenzothiazole, and add Paraformaldehyde, add dry HCl gas under reflux stirring for 1 to 2 hours, use TCL to track the reaction progress, cool to room temperature after the reaction, wash the organic layer with saturated NaCl until neutral, dry over anhydrous Na2SO4, filter, and rotary evaporate The solvent was removed to obtain a crude product, which was subjected to silica gel G column chromatography [eluent was v (ethyl acetate): v (petroleum ether) = 2:1] to obtain the fine compound 2-chloromethylthiobenzothiazole. Next, according to the first synthesis method, benthiocyanine (benthiocyanine) can be obtained.
Main reference materials
[1] Benthiocyanide
[2] CN201510561674.3 A single gram of benthiocyanine (benthiocyanine)Longan antibody hybridoma cell lines and their applications

 微信扫一扫打赏
微信扫一扫打赏

