Background and overview[1]
4-(4-Methylpiperazinemethyl)benzoyl chloride dihydrochloride can be used as a pharmaceutical synthesis intermediate. If 4-(4-methylpiperazinemethyl)benzoyl chloride dihydrochloride is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse skin thoroughly with soap and water. , if you feel discomfort, seek medical attention; if the eye contact occurs, separate the eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.

Preparation[1]

Put 1g of imacic acid (5g, 0.021mol, Anaiji Chemical) into a 100mL three-necked flask, add sulfonyl chloride (35mL) and catalyst N,N-dimethylformamide (0.3mL), and heat to reflux React for 22 hours, lower to room temperature, filter with suction, wash with n-hexane (10mL×2), and vacuum dry at 45-50°C for 2 hours to obtain white solid 4-(4-methylpiperazinemethyl)benzoyl chloride. Hydrochloride (1h) 5.1g, yield 95%.
Apply[1]
4-(4-methylpiperazinemethyl)benzoyl chloride dihydrochloride can undergo the following reactions:
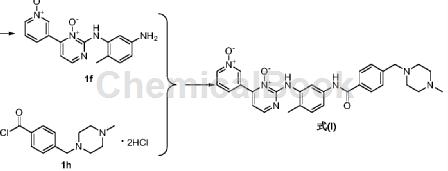
Put 1f (1.83g, 0.006mol) into a 100mL three-necked flask, add purified water (31mL), stir to lower the internal temperature to 0~3°C, and add 4-(4-methylpiperazinemethyl)benzene in batches Formyl chloride dihydrochloride (3.9g, 0.015mol), stir at room temperature for 2 hours, filter, add acetone (16mL) to the filtrate, heat to 45-50°C, add ammonia dropwise until the cloud point appears, cool to room temperature, stir and precipitate Crystallize for 2 hours, filter with suction, wash with purified water (10mL×2), and vacuum dry at 75-80°C for 2 hours to obtain 4-[(4-methyl-1-piperazinyl)methyl]- as a yellow solid N-[4-methyl-3-[[4-(1-oxo-3-pyridyl)-3-oxo-2-pyrimidinyl]amino]phenyl]benzamide formula (I) 2.29g, collected The rate is 73%, HPLC: 99.2%.
1HNMR (DMSO-d6, 500MHz): δ2.17(s, 3H), δ2.30(s, 3H), δ2.35( m, 8H), δ3.55 (s, 2H), δ7.31 (d, 1H), δ7.50 (m, 4H), δ7.66 (d, 1H), δ8.00 (d, 2H), δ8.18(d, 1H), δ8.28(d, 1H), δ8.63(s, 1H), δ8.75(d, 1H), δ8.89(s, 1H), δ9.75(s , 1H), δ10.26 (s, 1H).
Main reference materials
[1] CN107573322 Imatinib bis-nitroxide, its preparation method and use


 微信扫一扫打赏
微信扫一扫打赏

