Background and overview[1]
2,4-Dichlorophenylhydrazine is an important chemical raw material. The current preparation methods of 2,4-dichlorophenylhydrazine cannot avoid operations such as diazotization, sulfonation, and reduction of 2,4-dichloroaniline. These operations have high requirements on reaction temperature, and diazotization must be Low-temperature reaction, and poor control of the dripping speed and reaction degree of the sodium nitrite solution will lead to the formation of by-products and will also affect the following reduction reaction. The process route using sodium sulfite or sodium bisulfite as the reducing agent is relatively mature and has been widely used in the industrial production of 2,4-dichlorophenylhydrazine. However, it has many control conditions, complex process, and cumbersome operations. If hydrogen is needed, The pH value of the sodium oxide solution is adjusted to 6.5~7. If the control is not good, asphalt-like by-products will be produced, and the reaction temperature needs to be adjusted multiple times during the entire process.
Apply[1-4]
2,4-Dichlorophenylhydrazine is an important chemical raw material, mainly used in the fields of medicine, pesticides, dyes and photosensitive materials. It is an important intermediate in the synthesis of the weight loss drug rimonabant. Examples of its application are as follows:
1) Preparation of rimonabant hydrochloride: 4-chlorobenzaldehyde is condensed with nitroethane under alkali catalysis to obtain 1-p-chlorophenyl-2-nitropropene (II); D-glucose and 2 , 4-dichlorophenylhydrazine reacts to obtain phenylhydrazone compound (III); 1-p-chlorophenyl-2-nitropropene (II) and phenylhydrazone compound (III) react under alkaline conditions to obtain pyrazole compound (IV); Using KMnO4 as the catalyst, the pyrazole compound (IV) is oxidized in an alkaline solution of NaIO4 to obtain the carboxylic acid compound (V); the carboxylic acid compound (V) is acid chlorinated with a chlorine reagent and reacts with N-aminopiperidine The reaction produces an amide and forms a salt to obtain rimonabant hydrochloride (I). The synthesis route of the invention is reasonable, the raw materials are cheap, the reaction conditions are mild, the total yield is high, the quality of the reaction intermediate is easy to control, the product has industrial production potential, and the product has high purity and stable quality.
2) Preparation of 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxylate. Inorganic lithium salts and cheap organic base alkoxy metal compounds are used to effectively implement the Claisen condensation reaction of p-chloropropiophenone and oxalic acid diester to generate the corresponding stable 4-(4-chlorophenyl)-3-methyl- Enol complex lithium salt of 2,4-dioxobutyrate; the lithium salt is directly condensed with 2,4-dichlorophenylhydrazine hydrochloride in acetic acid, and then in the presence of the dehydrating agent thionyl chloride The cyclization reaction is completed in one pot to prepare 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxylate. The invention has the advantages of reasonable design of synthesis process; no use of expensive organic lithium reagents; shortened reaction time; high product yield, simple operation, low synthesis cost, and easy industrial-scale production.
3) Prepare a high-efficiency lithium battery electrolyte, including the following components by weight: 21-25 parts by weight of dimethyl sulfoxide, 1-5 parts by weight of hydroxyurea, and 25-31 parts by weight of dimethyl furan Parts by weight, 5-11 parts by weight of pyrrolidone, 15-21 parts by weight of potassium methylsulfonimide, 1-5 parts by weight of lithium phosphate, 1-5 parts by weight of 2,4-dichlorophenylhydrazine hydrochloride, carboxymethyl 1-5 parts by weight of sodium cellulose, 1-5 parts by weight of polyaspartic acid sodium salt, and 1-5 parts by weight of p-phenylenediamine oxalate. The invention provides a high-efficiency lithium battery electrolyte, so that the usage capacity of the lithium battery can reach more than 85% of its theoretical capacity.
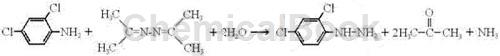
Preparation[1]
Put 162g of 2,4-dichloroaniline and 168g of acetonazine into a four-necked flask with a stirring, dropping funnel, thermometer and distillation column. The upper part of the receiver of the distillation column is connected with a conduit to absorb ammonia. gas; start stirring, heat the solution to 120~130°C, slowly add 54ml of water into the four-necked flask, control the reaction temperature to 100°C~130°C, when the gas generated by the reaction enters the rectification column, Acetone is extracted and ammonia is recovered at a top temperature of 54°C to 58°C. The water vapor and acetonazine entering the distillation column are condensed and flow back to the flask to continue participating in the reaction; after the water is added dropwise, the heat preservation reaction is continued. When the distillation column The reaction is completed when no ammonia gas is released from the receiver. All water and acetonitrile in the flask are evaporated under negative pressure. The solid matter in the flask is washed with absolute ethanol and dried to obtain 168.2g of 2,4-dichlorophenylhydrazine. .
Main reference materials
[1] CN201710375740.7 A preparation method of 2,4-dichlorophenylhydrazine
[2] CN201110089454.7 Synthesis method of rimonabant hydrochloride
[3] CN200810038071.5 Preparation method of 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxylate
[4] CN201711188590.5 A high-efficiency lithium battery electrolyte

 微信扫一扫打赏
微信扫一扫打赏

