Background and overview[1]
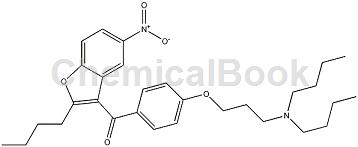
Synthesis of (2-butyl-5-nitro-3-benzofuryl)[4-[3-(dibutylamino)propoxy]phenyl]methanone as dronedarone hydrochloride intermediate. Dronedarone hydrochloride, chemical name: N-(2-butyl-3-(4-(3-dibutylaminopropoxy)benzoyl)benzofuran-5-yl)methanesulfonamide hydrochloride Salt is an antiarrhythmic drug.
Preparation[1]
The preparation of (2-butyl-5-nitro-3-benzofuryl)[4-[3-(dibutylamino)propoxy]phenyl]methanone is as follows:
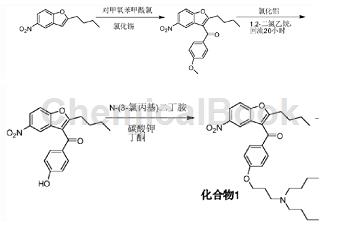
The specific steps are as follows: at room temperature, put in butanone (240L), potassium carbonate (17.9kg), 2-butyl-3-(4-hydroxybenzoyl)-5-nitrobenzofuran (40.0kg) ) and N-(3-chloropropyl)dibutylamine (26.6kg) into the reaction kettle, stir and heat to 75~81°C, keep the reaction for 9 hours, then cool to room temperature, filter, and wash with ethyl ketone (80L). Collect the filtrate. The solvent was removed by vacuum distillation below 50°C. After cooling to room temperature, ethyl acetate (200L) and water (200L) were added. After stirring for 15 minutes, let it stand for 30 minutes. The lower aqueous phase was separated and discarded, and the organic phase was retained. Add activated carbon (1.0kg), stir for 30 minutes, filter, wash with ethyl acetate (40L), and remove the solvent by vacuum distillation below 45°C.
Put in ethyl acetate (280L), adjust the pH value to 4.5~4.8 with glacial acetic acid, and unload the reaction materials into a clean container. At room temperature, add palladium-carbon suspension (dissolve 6.0kg palladium-carbon in 20L ethyl acetate), cool to 15-20°C, and add vacuum to make the vacuum in the reaction kettle reach 600mmHg. Fill in hydrogen at about 18°C to bring the pressure in the reactor to 1~3kg/cm2, stir for about 25 minutes, continue to add hydrogen until the pressure in the reactor reaches 3~5kg/cm2 sup>2, keep the reaction for 2 hours and then close the hydrogen valve, release the hydrogen into the pool, introduce nitrogen so that the pressure in the reactor reaches 1.5~2kg/cm2, and stir for 5 minutes. Releases nitrogen gas into the pool.
Filter under pressure (1.5~2kg/cm2nitrogen pressure), collect the filtrate into a container containing 25% ammonia solution (30L ammonia dissolved in 300L water), release nitrogen, and use acetic acid to Wash with ethyl ester (120L), collect the filtrate and combine. Pour the filtrate into the reaction kettle, stir for 20 minutes, let it stand for about 30 minutes, separate, and discard the water phase. At room temperature, add 25% ammonia solution (30L ammonia dissolved in 300L water) into the organic phase, stir for about 15 minutes, let stand for 30 minutes, separate, and discard the water phase. At room temperature, add sodium chloride solution (40kg sodium chloride dissolved in 200L water) into the organic phase, stir for about 15 minutes, let stand for 30 minutes, separate, and discard the water phase. Ethyl acetate was distilled off at about 30°C, ethyl acetate (60L) was added, ethyl acetate was continued to be distilled off at about 30°C, methanol (40L) was added, and methanol was removed by vacuum distillation at about 30°C.
At room temperature, add methanol (378L) to the reaction kettle, heat to about 35°C, add oxalic acid dihydrate (32.2kg), heat to 50°C, keep stirring for 1.5 to 2.0 hours, cool to about 30°C, and keep warm. Stir the reaction for 2.0 to 2.5 hours. Centrifuge and spin-dry until the mother liquor is removed. Wash the wet filter cake with methanol (108L) and spin-dry until the mother liquor is removed. Unload the wet filter cake into the vacuum drying box, add vacuum to 600mmHg, then heat up to about 50°C and dry for 4 hours. Yield: about 80.0%.
Main reference materials
[1] CN106892886 A kind of preparation method of dronedarone hydrochloride

 微信扫一扫打赏
微信扫一扫打赏

