Background and overview[1]
2-Bromo-1-(4-morpholinophenyl)-1-ethanone can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
The preparation of 2-bromo-1-(4-morpholinophenyl)-1-ethanone is as follows:
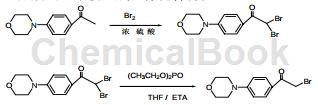
1) Preparation of intermediate 4-morpholinophenylethanone
Take 50g of 4-fluoroacetophenone, dissolve it in 350mL of dimethyl sulfoxide (DMSO) (about 7 to 8 times mass to volume ratio), stir evenly, and it will become a colorless solution. Slowly add 78.8g (0.9mol) of morpholine dropwise at room temperature. After the dripping is complete, raise the temperature to 120°C and reflux. The solution gradually turned light yellow. React for 3 to 4 hours, and use TLC to monitor the reaction end point. The reaction is complete. After the reaction is completed, let it stand to room temperature. Slowly pour the reaction solution into a large amount of ice water, stir, and a yellow solid will precipitate. After suction filtration, collect the filter cake, wash it with water twice, and dry it to obtain 63 g of yellow solid (yield 85%).
2) Preparation of intermediate 2,2-dibromo-1-(4-morpholinophenyl)ethanone
Add 30g (0.15mol) of 4-morpholinophenylethanone and 210mL of concentrated sulfuric acid (about 7 to 8 times the mass to volume ratio) into a 500mL three-necked flask equipped with mechanical stirring and a thermometer, and stir until Dissolve. Cool the reaction solution to 0°C, slowly drop 7.8 mL of bromine at this temperature, and control the temperature at 0°C. After the dripping is completed, react at room temperature. The reaction took about 6 hours, and TLC was used to monitor whether the reaction was complete. Pour the reaction solution into a large amount of ice water. If a yellow-green solid precipitates, stir. After the ice dissolves, filter and wash with water until neutral. The filter cake was collected and dried to obtain 47g of yellow-green solid, with a yield of 88%.
3) Preparation of compound 2-bromo-1-(4-morpholinophenyl)-1-ethanone
Add 30g of 2,2-bromo-1-(4-morpholinophenyl)ethanone (0.08mol) and about 160mL of tetrahydrofuran (5 to 6 times mass to volume ratio) into a 500mL three-neck flask, stir and cool to 0℃. Slowly add a mixed solution of 11.1 mL of diethyl phosphite, 12 mL of triethylamine, and 70 mL of tetrahydrofuran dropwise, keeping the temperature at 0°C. After the dripping is completed, the reaction takes place at room temperature for about 5 hours. The reaction endpoint was monitored using TLC and the reaction was complete. The reaction solution was suction filtered to obtain a slightly yellow solid (containing a very small amount of product), most of which were impurities. Collect the filtrate and concentrate to about 20 mL. Pour the concentrated solution into a large amount of ice water. If a yellow-green solid precipitates, continue stirring until all the ice cubes melt. Filter with suction, wash the filter cake with water, and dry to obtain 20.1g of yellow-green product 2-bromo-1-(4-morpholinophenyl)-1-ethanone, with a yield of 85.5%.
Main reference materials
[1] Design and synthesis of cyclin-dependent kinase inhibitors (CDKs) intermediates

 微信扫一扫打赏
微信扫一扫打赏

