Background and overview[1]
Heterocyclic compounds, especially natural products containing furan and thiophene structures, mostly have good biological activity. Therefore, how to synthesize heterocyclic molecules containing furan and thiophene skeletons with high chemical selectivity and stereoselectivity is particularly important. Enyne compounds are often used to synthesize complex organic compounds due to their good reactivity and multiple reaction sites. In recent years, chemists have synthesized 2,5-disubstituted furans such as 2,5-diphenylfuran and thiophene compounds through transition metal catalysis of alkenes. They mainly use transition metals such as gold, ruthenium, palladium and copper to complete the ring. chemical reactions, such as palladium catalysis or direct use of potassium hydroxide to promote the synthesis of symmetric 2,5-disubstituted furans, thiophenes and pyrrole derivatives of alkynes with high yields.
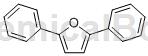
Preparation[1]
The preparation of 2,5-diphenylfuran is as follows: add a stirring magnet, terminal alkyne (1mmol), DMAc (2mL), CuI (5mol%) and TMEDA (10mol%) to the reaction tube in sequence, and then use The balloon was filled with oxygen (101kPa), and the reaction was carried out at 30°C for 6 hours; then KOH (2mmol) was added, the temperature was raised to 90°C, and the reaction was stirred until the complete conversion of the raw materials was monitored by thin layer chromatography. The sample was directly mixed with silica gel and purified by column chromatography, using petroleum Ether was used as eluent to obtain the product 2,5-diphenylfuran. Light yellow solid, yield 91%.
m.p.66~68℃ (lit.68~70℃); 1HNMR (400MHz, CDCl3) δ: 7.74(d, J=7.6Hz, 4H), 7.41~7.38(m, 4H), 7.28~7.23( m, 2H), 6.73 (s, 2H); 13CNMR (100MHz, CDCl3) δ: 153.5, 130.9, 128.8, 127.5, 123.9, 107.4;
MS (EI, 70eV) m/z (%): 220, 207, 191, 165, 115, 77.
Main reference materials
[1] Li Yibiao, Cheng Liang, Chen Lu, et al. One-pot synthesis of substituted thiophene and furan compounds based on terminal alkynes [J]. Organic Chemistry, 2016, 36(10): 2426-2436.

 微信扫一扫打赏
微信扫一扫打赏

